Question
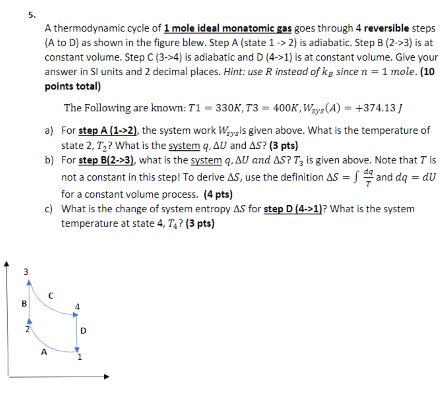
A thermodynamic cycle of 1 mole ideal monatomic gas goes through 4 reversible steps (A to D ) as shown in the figure blew.
\ A thermodynamic cycle of 1 mole ideal monatomic gas goes through 4 reversible steps\ (A to
D) as shown in the figure blew. Step
A(state
1->2) is adiabatic. Step
B(2->3)is at\ constant volume. Step
C(3->4)is adiabatic and
D(4->1)is at constant volume. Give your\ answer in SI units and 2 decimal places. Hint: use
Rinsteod of
k_(B)since
n=1mole. (10\ points total)\ The Following are known:
T1=330K,T3=400K,W_(sys )(A)=+374.13J\ a) For
stepA(1->2), the system work
W_(sys)is given above. What is the temperature of\ state
2,T_(2)? What is the system
q,\\\\Delta Uand
\\\\Delta S? (3 pts)\ b) For step
B(2->3), what is the system
q,\\\\Delta Uand
\\\\Delta S?
T_(3)is given above. Note that
Tis\ not a constant in this step! To derive
\\\\Delta S, use the definition
\\\\Delta S=\\\\int (dq)/(T)and
dq=dU\ for a constant volume process. (4 pts)\ c) What is the change of system entropy
\\\\Delta Sfor step
D(4->1)? What is the system\ temperature at state
4,T_(4)? (3 pts)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


