Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A tiny sample of an aqueous solution of two substances R and P is sketched below, as if it was under an imaginary microscope so
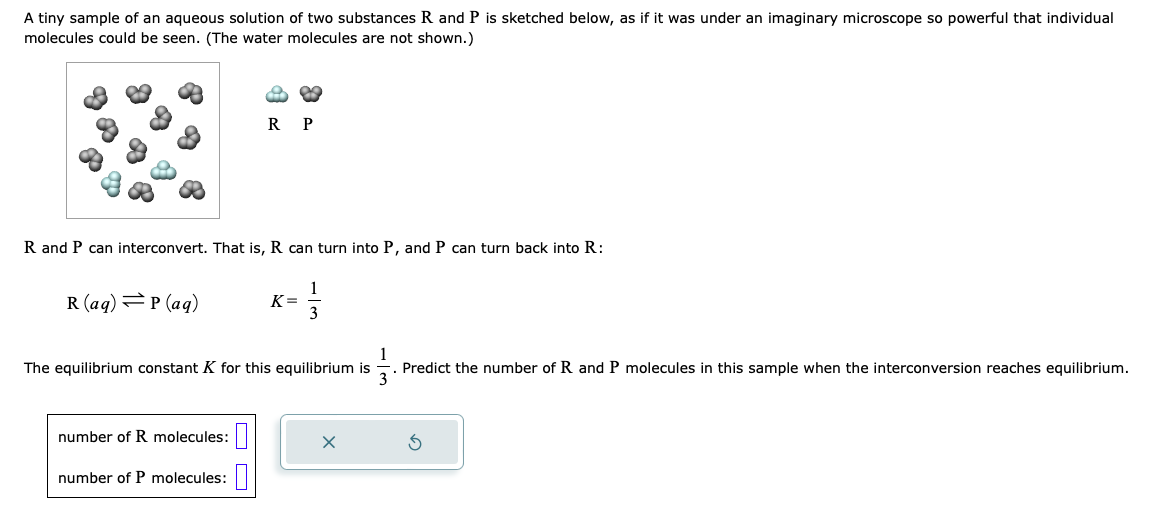
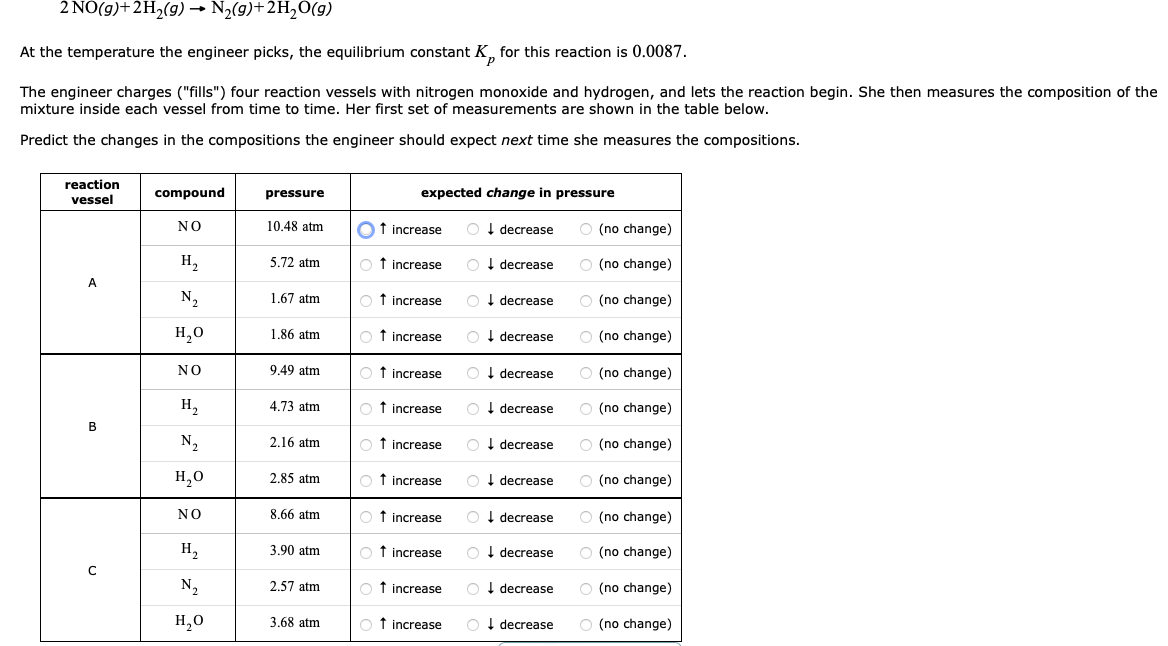 A tiny sample of an aqueous solution of two substances R and P is sketched below, as if it was under an imaginary microscope so powerful that individual molecules could be seen. (The water molecules are not shown.) RP R and P can interconvert. That is, R can turn into P, and P can turn back into R : R(aq)P(aq)K=31 The equilibrium constant K for this equilibrium is 31. Predict the number of R and P molecules in this sample when the interconversion reaches equilibrium. number of R molecules: number of P molecules: 2NO(g)+2H2(g)N2(g)+2H2O(g) At the temperature the engineer picks, the equilibrium constant Kp for this reaction is 0.0087 . The engineer charges ("fills") four reaction vessels with nitrogen monoxide and hydrogen, and lets the reaction begin. She then measures the composition of the mixture inside each vessel from time to time. Her first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time she measures the compositions
A tiny sample of an aqueous solution of two substances R and P is sketched below, as if it was under an imaginary microscope so powerful that individual molecules could be seen. (The water molecules are not shown.) RP R and P can interconvert. That is, R can turn into P, and P can turn back into R : R(aq)P(aq)K=31 The equilibrium constant K for this equilibrium is 31. Predict the number of R and P molecules in this sample when the interconversion reaches equilibrium. number of R molecules: number of P molecules: 2NO(g)+2H2(g)N2(g)+2H2O(g) At the temperature the engineer picks, the equilibrium constant Kp for this reaction is 0.0087 . The engineer charges ("fills") four reaction vessels with nitrogen monoxide and hydrogen, and lets the reaction begin. She then measures the composition of the mixture inside each vessel from time to time. Her first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time she measures the compositions Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


