Question
A titration of 2.00 ml of hydrogen peroxide solution (Mw = 34.01 g/ mol) used 35.57 ml of 0.1 N (0.02 M) potassium permanganate
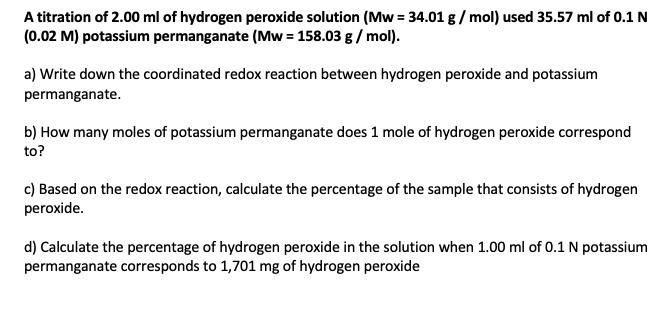
A titration of 2.00 ml of hydrogen peroxide solution (Mw = 34.01 g/ mol) used 35.57 ml of 0.1 N (0.02 M) potassium permanganate (Mw = 158.03 g/ mol). a) Write down the coordinated redox reaction between hydrogen peroxide and potassium permanganate. b) How many moles of potassium permanganate does 1 mole of hydrogen peroxide correspond to? c) Based on the redox reaction, calculate the percentage of the sample that consists of hydrogen peroxide. d) Calculate the percentage of hydrogen peroxide in the solution when 1.00 ml of 0.1 N potassium permanganate corresponds to 1,701 mg of hydrogen peroxide
Step by Step Solution
3.31 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
2mL Ho2 33 57ML of O1N KMnou MW 341olg Ime 2 KMnou 45Hg 2Mn Soy ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Microeconomics
Authors: Douglas Bernheim, Michael Whinston
2nd edition
73375853, 978-0073375854
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



