Question
Benzoic acid is a weak acid that has antimicrobial properties. Its sodium salt, sodium benzoate, is a preservative found in foods, medications, and personal
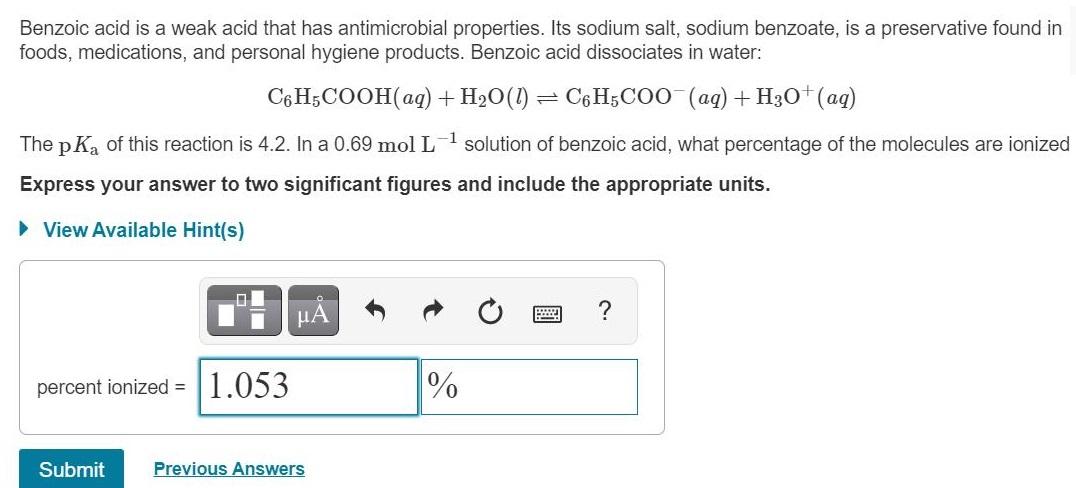
Benzoic acid is a weak acid that has antimicrobial properties. Its sodium salt, sodium benzoate, is a preservative found in foods, medications, and personal hygiene products. Benzoic acid dissociates in water: C6 H;COOH(aq) +H2O(1) - C6 H5COO (aq) + H30+(aq) The p K, of this reaction is 4.2. In a 0.69 mol L 1 solution of benzoic acid, what percentage of the molecules are ionized Express your answer to two significant figures and include the appropriate units. View Available Hint(s) A percent ionized = 1.053 % Submit Previous Answers
Step by Step Solution
3.55 Rating (145 Votes )
There are 3 Steps involved in it
Step: 1
B 6HC COOM 1 69 M M M 069...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



