Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A to D please 2) Consider the energy transfer between a can of soda and the water in a cooler (you can assume that the
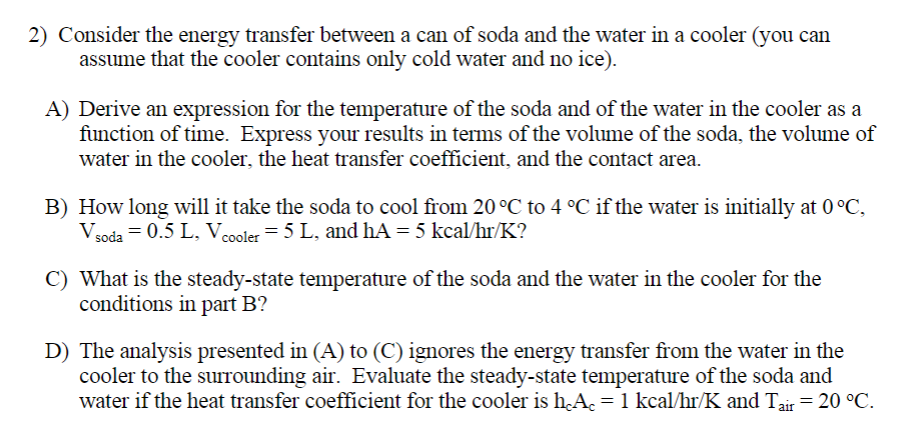
A to D please
2) Consider the energy transfer between a can of soda and the water in a cooler (you can assume that the cooler contains only cold water and no ice). A) Derive an expression for the temperature of the soda and of the water in the cooler as a function of time. Express your results in terms of the volume of the soda, the volume of water in the cooler, the heat transfer coefficient, and the contact area. B) How long will it take the soda to cool from 20C to 4C if the water is initially at 0C, Vsoda=0.5L,Vcooler=5L, and hA=5kcal/hr/K ? C) What is the steady-state temperature of the soda and the water in the cooler for the conditions in part B? D) The analysis presented in (A) to (C) ignores the energy transfer from the water in the cooler to the surrounding air. Evaluate the steady-state temperature of the soda and water if the heat transfer coefficient for the cooler is hcAc=1kcal/hr/K and Tair=20CStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


