Question
a) To determine boiling point of liquids, we need to look at both intramolecular and intermolecular forces of attraction. Name the intra- and inter-molecular
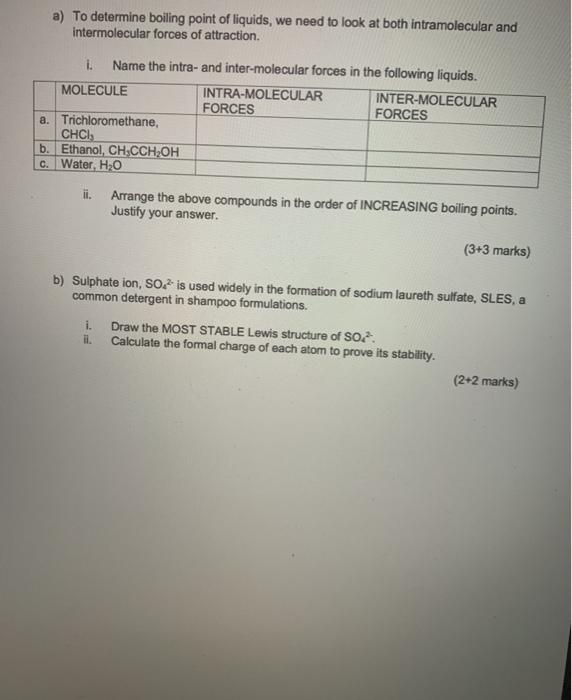
a) To determine boiling point of liquids, we need to look at both intramolecular and intermolecular forces of attraction. Name the intra- and inter-molecular forces in the following liquids. INTRA-MOLECULAR INTER-MOLECULAR MOLECULE a. Trichloromethane, CHCI b. Ethanol, CH,CCHOH c. Water, HO ii. FORCES FORCES Arrange the above compounds in the order of INCREASING boiling points. Justify your answer. (3+3 marks) b) Sulphate ion, SO is used widely in the formation of sodium laureth sulfate, SLES, a common detergent in shampoo formulations. i. Draw the MOST STABLE Lewis structure of SO ili. Calculate the formal charge of each atom to prove its stability. (2+2 marks)
Step by Step Solution
3.38 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamental Statistics for the Behavioral Sciences
Authors: David C. Howell
8th Edition
1285076915, 978-1285076911
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



