Question
A to P second order liquid phase reaction is to be carried out in a jacketed batch reactor for 80% conversion. Initial A composition of
A to P second order liquid phase reaction is to be carried out in a jacketed batch reactor for 80% conversion. Initial A composition of 4 m3 reaction mixture will be 2.3 mol/L. a) Find the necessary reaction time and heat transfer fluid temperature profile (Ts=f(t)) for T=363 K isothermal operation. b) Find the necessary reaction time and temperature profile of the reaction mixture (T=f(t)) under adiabatic conditions, if the initial temperature is To=340 K. c) Discuss all your results and comment on the OTP for this reaction. DATA 1- Heat of reaction : - Delta H= 12,000 cal/mol 2- Heat transfer coefficient for the jacketed reactor: U =130 cal/dm2minoC 3-Avarege physical properties of the mixture: CP =0.95 cal/goC , density =1.02 g/cm3
4- Design graph for the reaction (CA0=2.3 mol/L, Tmax=370 K; rate units [=] mol/Lh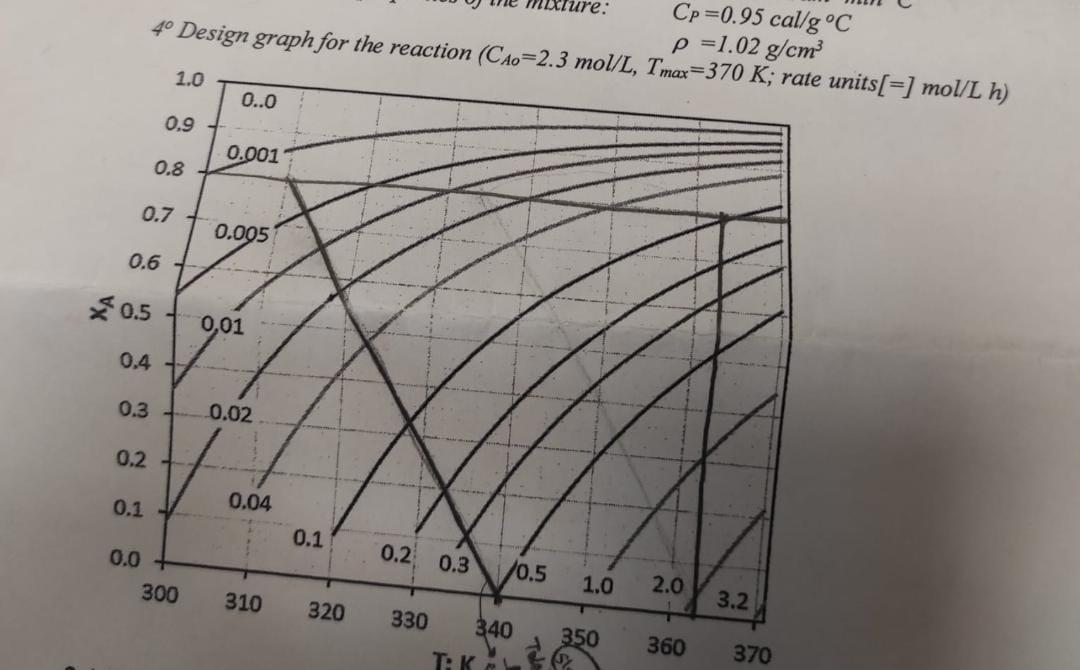
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


