Question
A uranium ion and an iron ion are separated by a distance of R = 29.90 nm, as shown in the figure. The uranium
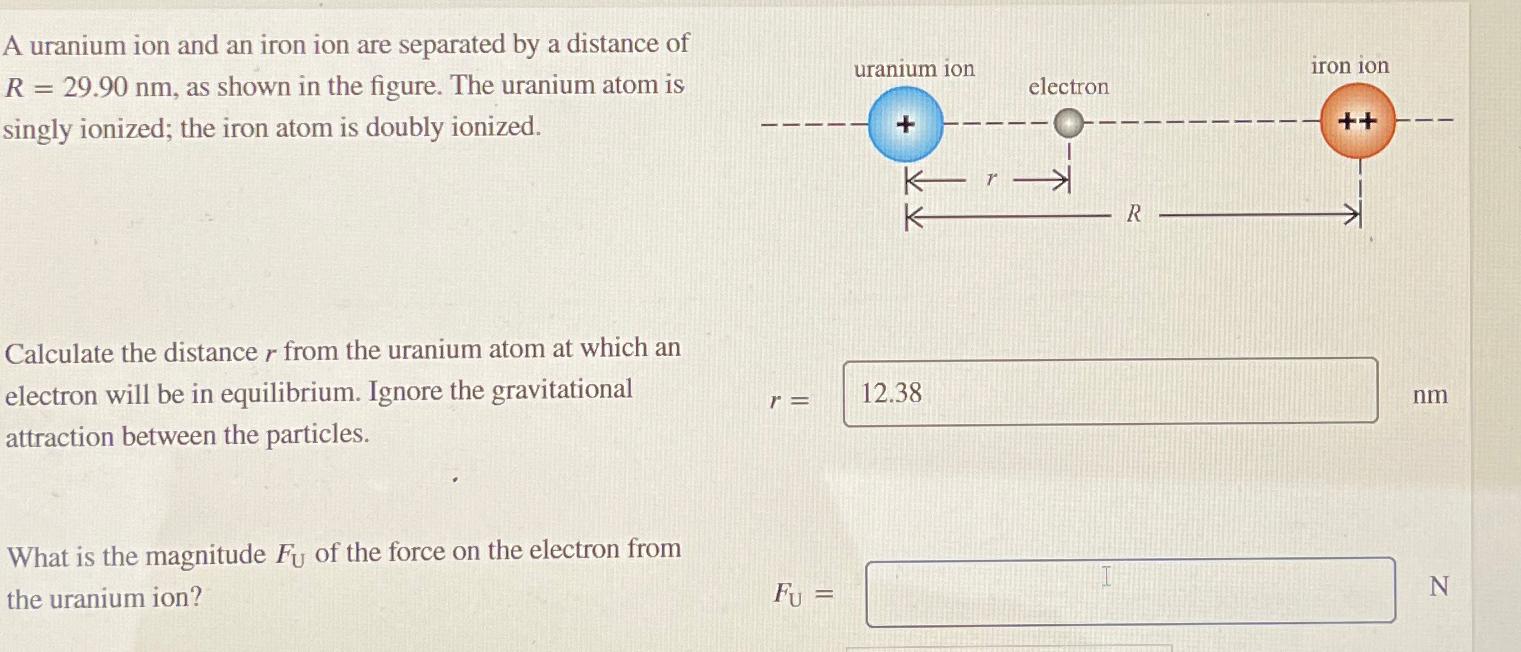
A uranium ion and an iron ion are separated by a distance of R = 29.90 nm, as shown in the figure. The uranium atom is singly ionized; the iron atom is doubly ionized. Calculate the distance r from the uranium atom at which an electron will be in equilibrium. Ignore the gravitational attraction between the particles. r = What is the magnitude Fu of the force on the electron from the uranium ion? uranium ion electron Kr K 12.38 R iron ion ++ nm N Fu =
Step by Step Solution
3.52 Rating (145 Votes )
There are 3 Steps involved in it
Step: 1
1 To calculate the distance from the uranium atom at which an electron will be in equilibrium ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Physics
Authors: Jearl Walker, Halliday Resnick
8th Extended edition
471758019, 978-0471758013
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



