Answered step by step
Verified Expert Solution
Question
1 Approved Answer
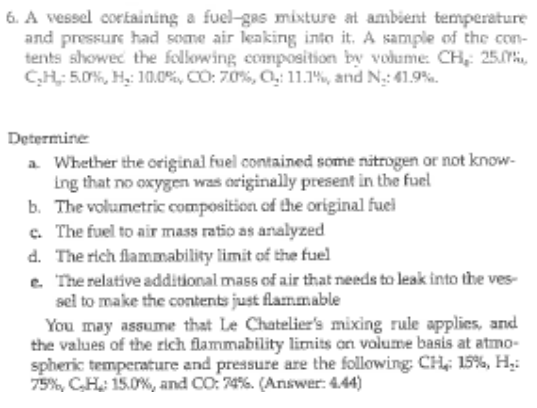
A vessel cortaining a fuel - gas mixture at ambient temperature and pressure had some air keaking into it . A sample of the con
A vessel cortaining a fuelgas mixture at ambient temperature
and pressure had some air keaking into it A sample of the con
tents showec the following composition by vodume: : ia
:::: and :
Determine
a Whether the original fuel contained some nitrogen or not know
Ing that no oxygen was originally present in the fuel
b The volumetric composition of the original fuel
c The fuel to air mass ratio as analyzed
d The rich flammability limit of the fuel
e 'The relative additional mass of air that needs to leak into the ves
sel to make the contents just flammable
You may assume that Le Chatelier's mixing rule applies, and
the values of the rich flammability limits on volume basis at atmo
spheric temperature and pressure are the following: : :
: and CO Answer:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


