A waste gas contains 55 mol % DMF (dimethylformamide a common solvent) in air. A purification unit is available that can remove a fraction
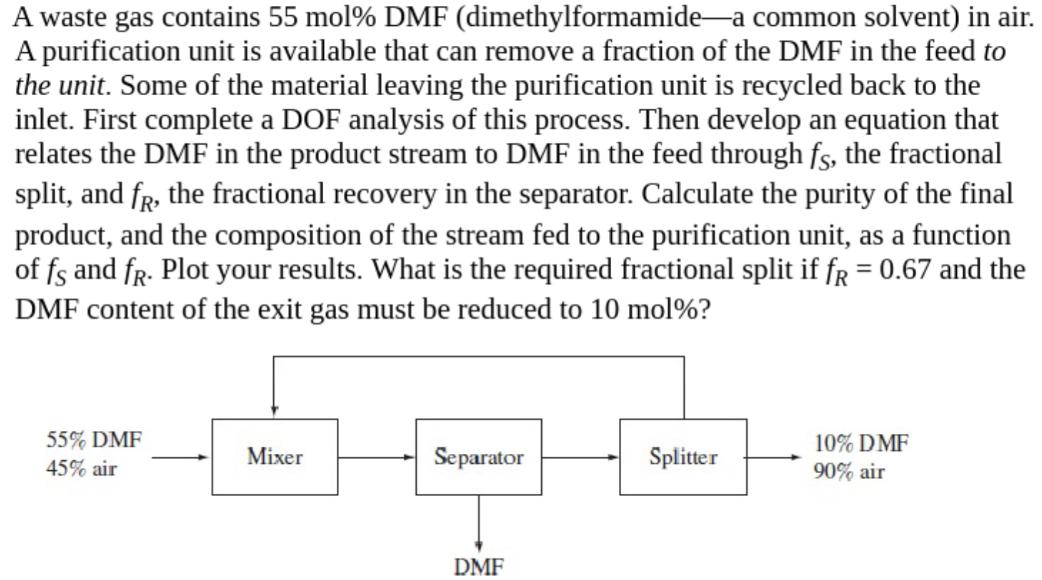
A waste gas contains 55 mol % DMF (dimethylformamide a common solvent) in air. A purification unit is available that can remove a fraction of the DMF in the feed to the unit. Some of the material leaving the purification unit is recycled back to the inlet. First complete a DOF analysis of this process. Then develop an equation that relates the DMF in the product stream to DMF in the feed through fs, the fractional split, and f, the fractional recovery in the separator. Calculate the purity of the final product, and the composition of the stream fed to the purification unit, as a function of fs and fr. Plot your results. What is the required fractional split if fR = 0.67 and the DMF content of the exit gas must be reduced to 10 mol%? 55% DMF 45% air Mixer Separator DMF Splitter 10% DMF 90% air
Step by Step Solution
There are 3 Steps involved in it
Step: 1
DOF Degrees of Freedom Analysis 1 Lets identify the variables x DMF content in the feed 55 mol y DMF ... View full answer

Get step-by-step solutions from verified subject matter experts
100% Satisfaction Guaranteed-or Get a Refund!
Step: 2Unlock detailed examples and clear explanations to master concepts

Step: 3Unlock to practice, ask and learn with real-world examples

See step-by-step solutions with expert insights and AI powered tools for academic success
-
 Access 30 Million+ textbook solutions.
Access 30 Million+ textbook solutions.
-
 Ask unlimited questions from AI Tutors.
Ask unlimited questions from AI Tutors.
-
 Order free textbooks.
Order free textbooks.
-
 100% Satisfaction Guaranteed-or Get a Refund!
100% Satisfaction Guaranteed-or Get a Refund!
Claim Your Hoodie Now!

Study Smart with AI Flashcards
Access a vast library of flashcards, create your own, and experience a game-changing transformation in how you learn and retain knowledge
Explore Flashcards





