Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A wet sulfuric acid reactor is fed with a tail gas stream consisting of 40,40 and 20mol% of H2S,CH4 and CO2, respectively at a total
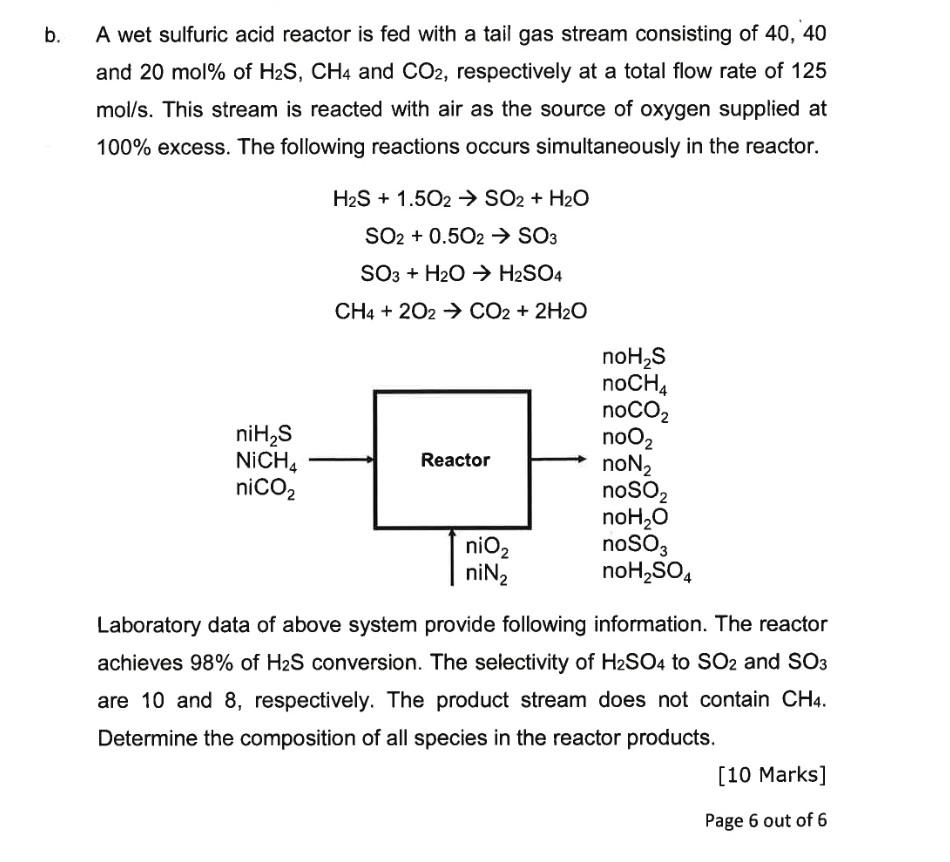
A wet sulfuric acid reactor is fed with a tail gas stream consisting of 40,40 and 20mol% of H2S,CH4 and CO2, respectively at a total flow rate of 125 mol/s. This stream is reacted with air as the source of oxygen supplied at 100% excess. The following reactions occurs simultaneously in the reactor. H2S+1.5O2SO2+H2OSO2+0.5O2SO3SO3+H2OH2SO4CH4+2O2CO2+2H2O Laboratory data of above system provide following information. The reactor achieves 98% of H2S conversion. The selectivity of H2SO4 to SO2 and SO3 are 10 and 8 , respectively. The product stream does not contain CH4. Determine the composition of all species in the reactor products
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


