Question: a) What is liquid-liquid separal b) Describe three distinct processes involved in liquid-liquid operatio c) Acetic acid in an aqueous solution containing 1.0% acetic
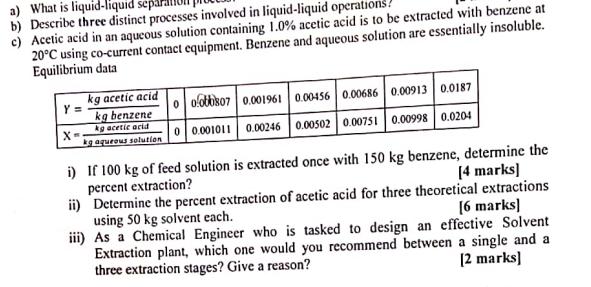
a) What is liquid-liquid separal b) Describe three distinct processes involved in liquid-liquid operatio c) Acetic acid in an aqueous solution containing 1.0% acetic acid is to be extracted with benzene at 20C using co-current contact equipment. Benzene and aqueous solution are essentially insoluble. Equilibrium data kg acetic acid 0 ootbiso7 0.001961 0.00456 0.00686 0.00913 0.0187 kg benzene kg acetic acid x- 0 0.001011 0.00246 0.00502| 0.00751 0.00998 0.0204 gaqurous solution i) If 100 kg of feed solution is extracted once with 150 kg benzene, determine the percent extraction? ii) Determine the percent extraction of acetic acid for three theoretical extractions using 50 kg solvent each. iii) As a Chemical Engineer who is tasked to design an effective Solvent Extraction plant, which one would you recommend between a single and a three extraction stages? Give a reason? (4 marks) [6 marks) [2 marks]
Step by Step Solution
3.38 Rating (154 Votes )
There are 3 Steps involved in it
Graph i x F 001 wt fraction acetic acid x F 0011001 00101 kg acetic acid kg aquoius solut... View full answer

Get step-by-step solutions from verified subject matter experts


