Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A. What is the amount of heat that must be removed in cooling 1 lb-mol of 0.8 Specific Gravity Natural Gas at 500 psia from
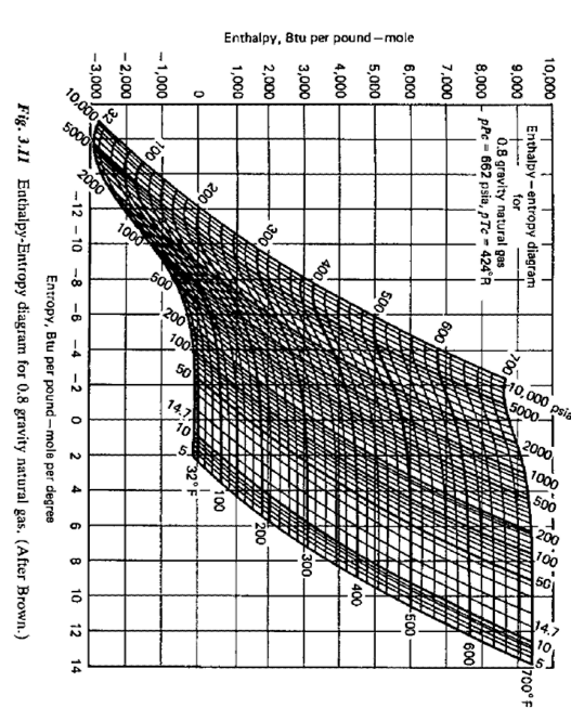
A. What is the amount of heat that must be removed in cooling 1 lb-mol of 0.8 Specific Gravity Natural Gas at 500 psia from 600F to 300F? (Use the chart)
B. If this gas is heated from 200F to 300F how much heat is required per lb-mol of gas?
Entropy, Btu per pound-mole per degree Fig. 3.II Enthalpy-Entropy diagram for 0.8 gravity natural gas. (After Brown.) Entropy, Btu per pound-mole per degree Fig. 3.II Enthalpy-Entropy diagram for 0.8 gravity natural gas. (After Brown.)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


