Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A. What is the value of the rate constant (k) for this reaction at this temperature? Express your answer in liters per mole second to
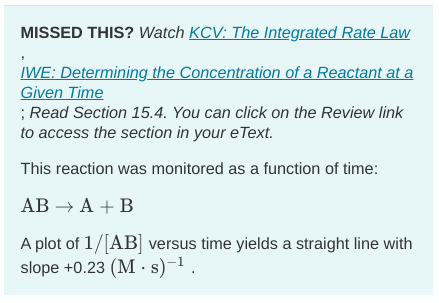
A. What is the value of the rate constant (k) for this reaction at this temperature? Express your answer in liters per mole second to two significant figures.
B. What is the half-life when the initial concentration is 0.50 M? Express your answer in seconds to two significant figures.
C. What is the half-life when the initial concentration is 0.50 M? Express your answer in seconds to two significant figures.
MISSED THIS? Watch KCV: The Integrated Rate Law IWE: Determining the Concentration of a Reactant at a Given Time ; Read Section 15.4. You can click on the Review link to access the section in your eText. This reaction was monitored as a function of time: ABA+B A plot of 1/[AB] versus time yields a straight line with slope +0.23(Ms)1Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


