Question
A worker splash fills hexane (MW: 86.18; TLV-TWA: 50 ppm) into a 2642-gallon (i.e., 10 m 3 volume) vessel through a 10-cm diameter filling hole.
A worker splash fills hexane (MW: 86.18; TLV-TWA: 50 ppm) into a 2642-gallon (i.e., 10 m3 volume) vessel through a 10-cm diameter filling hole. It takes about 2 hr to fill the vessel at a constant filling rate. The local ventilation rate is 150 m3/min, the ambient pressure and temperature are 1 atm (760 mm-Hg) and 25oC, respectively. Assume average non-ideal mixing factor k for the ventilation condition. The vapor pressure of hexane can be estimated using
Antoine equation with the given constants: 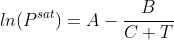 , where T is in K and Psat is the saturation pressure (mm Hg)
, where T is in K and Psat is the saturation pressure (mm Hg)
Antoine constants for hexane: A = 15.8366, B = 2697.55 and C = -48.78
Estimate the local concentration of the hexane in ppm. What observations you can make regarding workers exposure to hexane?
In(Psal) = A- B C+TStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


