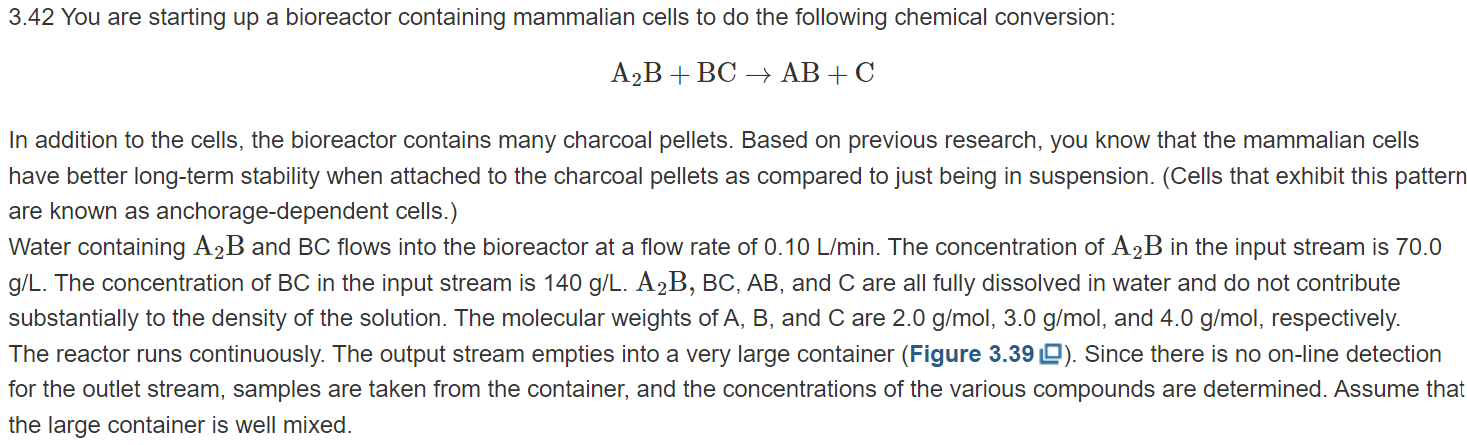
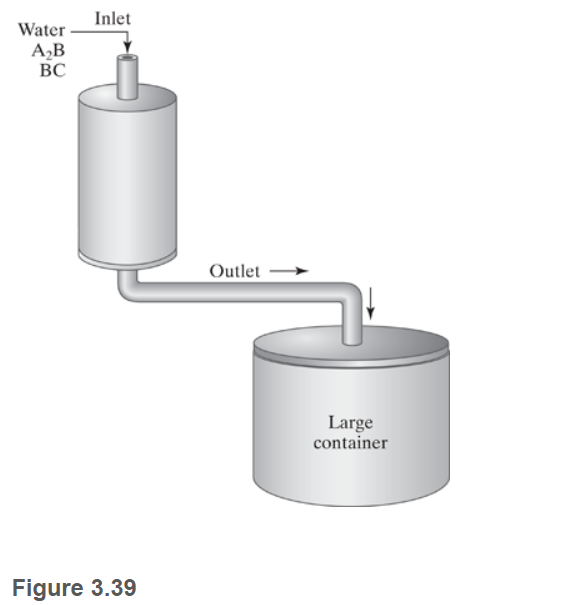

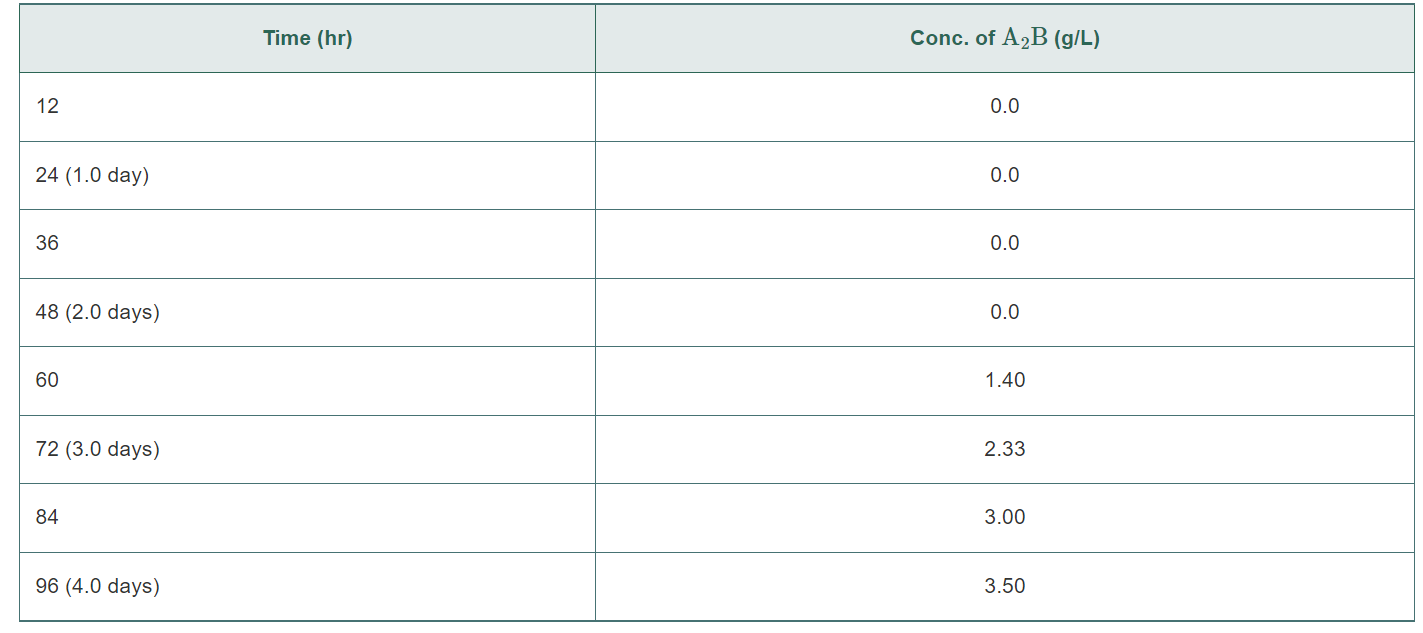
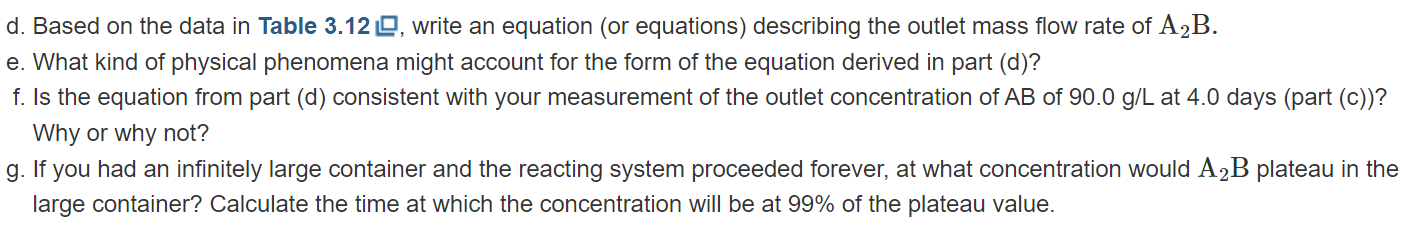
A2B+BCAB+C In addition to the cells, the bioreactor contains many charcoal pellets. Based on previous research, you know that the mammalian cells have better long-term stability when attached to the charcoal pellets as compared to just being in suspension. (Cells that exhibit this pattern are known as anchorage-dependent cells.) Water containing A2B and BC flows into the bioreactor at a flow rate of 0.10L/min. The concentration of A2B in the input stream is 70.0 g/L. The concentration of BC in the input stream is 140g/L.A2B,BC,AB, and C are all fully dissolved in water and do not contribute substantially to the density of the solution. The molecular weights of A,B, and C are 2.0g/mol,3.0g/mol, and 4.0g/mol, respectively. The reactor runs continuously. The output stream empties into a very large container (Figure 3.39 ). Since there is no on-line detection for the outlet stream, samples are taken from the container, and the concentrations of the various compounds are determined. Assume that the large container is well mixed. Figure 3.39 Sioreactor containing mammalian cells and charcoal pellets. The outlet empties into a large container. a. You run the bioreactor for 4.0 days. During that period, the entire outlet is captured in the large container; none is emptied. You sample the container after 4.0 days and determine that the concentration of A2B is 3.5g/L. Based on this information, determine the outlet flow rate of A2B. b. Determine the reaction rate, R, of the system. What are the fractional conversions of A2B and BC ? c. You manage to borrow an instrument to do on-line detection right at the end of your 4.0-day experiment. You sample the outlet stream (not the container) and determine that the outlet concentration of AB is 90.0g/L. Is this measurement consistent with your results from parts (a) and (b)? Why or why not? You decide to rerun the whole experiment. You throw out all the charcoal pellets and mammalian cells. You reload the bioreactor with fresh charcoal and cells. Before you begin this new experiment, you also empty out your large container that was capturing the outlet flow. During this run, you decide to sample from the large container every 12 hours. The concentrations of A2B are recorded in Table 3.12 Note that the large container is not emptied during the 4.0-day run. Instead, all of the outlet liquid is collected and mixed in the large container. TABLE 3.12 Samples of A2B Taken from Large Container \begin{tabular}{|l|c|} \hline \multicolumn{1}{|c|}{ Time (hr) } & Conc. of A2B (g/L) \\ \hline 12 & 0.0 \\ \hline 24(1.0 day) & 0.0 \\ \hline 36 & 0.0 \\ \hline 48(2.0 days) & 0.0 \\ \hline 60 & 1.40 \\ \hline 72 (3.0 days) & 3.33 \\ \hline 84 & 3.00 \\ \hline 96(4.0 days) & 3.50 \\ \hline \end{tabular} d. Based on the data in Table 3.12 , write an equation (or equations) describing the outlet mass flow rate of A2B. e. What kind of physical phenomena might account for the form of the equation derived in part (d)? f. Is the equation from part (d) consistent with your measurement of the outlet concentration of AB of 90.0g/L at 4.0 days (part (c))? Why or why not? g. If you had an infinitely large container and the reacting system proceeded forever, at what concentration would A2B plateau in the large container? Calculate the time at which the concentration will be at 99% of the plateau value











