Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A=8 B=3 Biodegradable polymers have found increasing use in the medical field because they have the advantage of harmlessly breaking down in the body after
 A=8
A=8
B=3
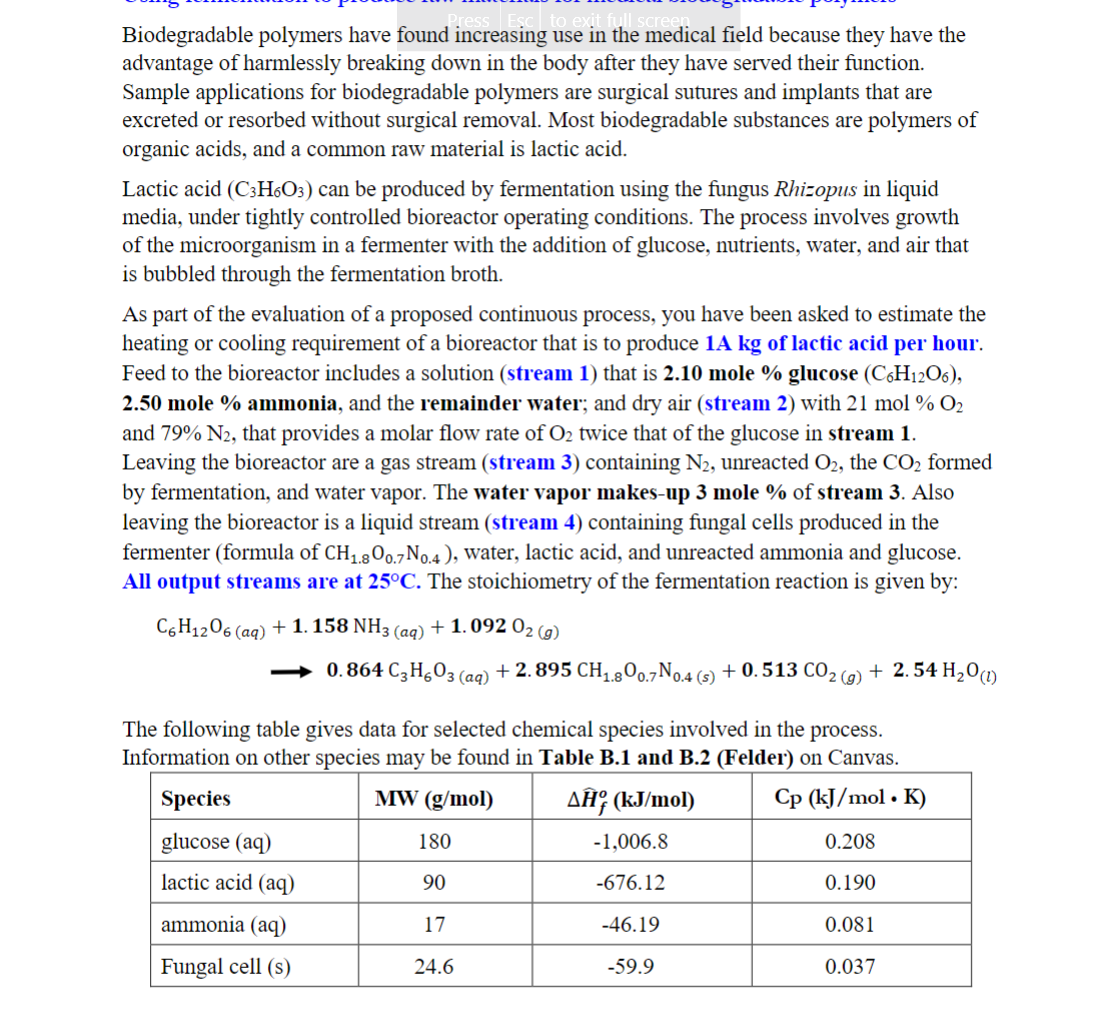
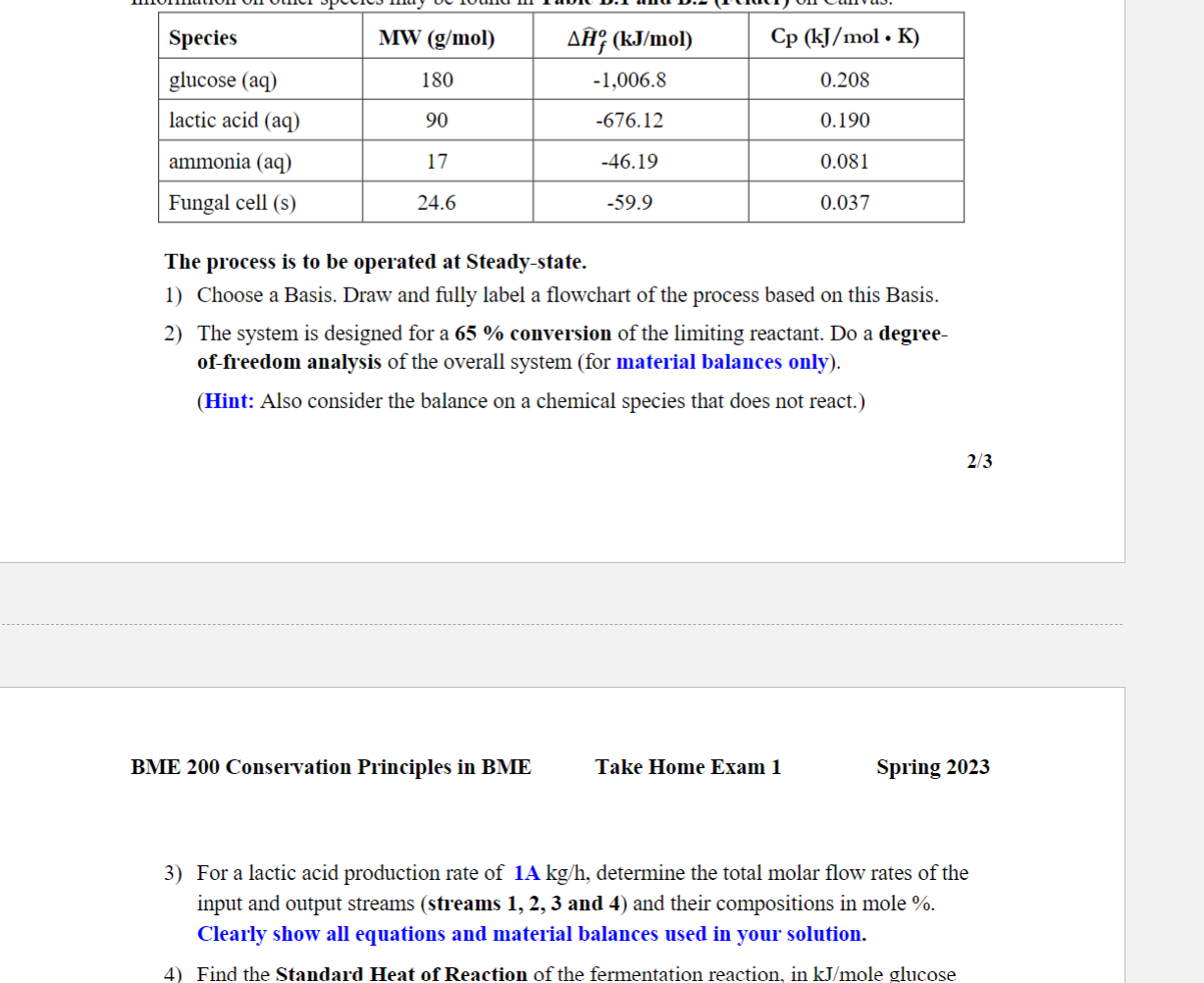
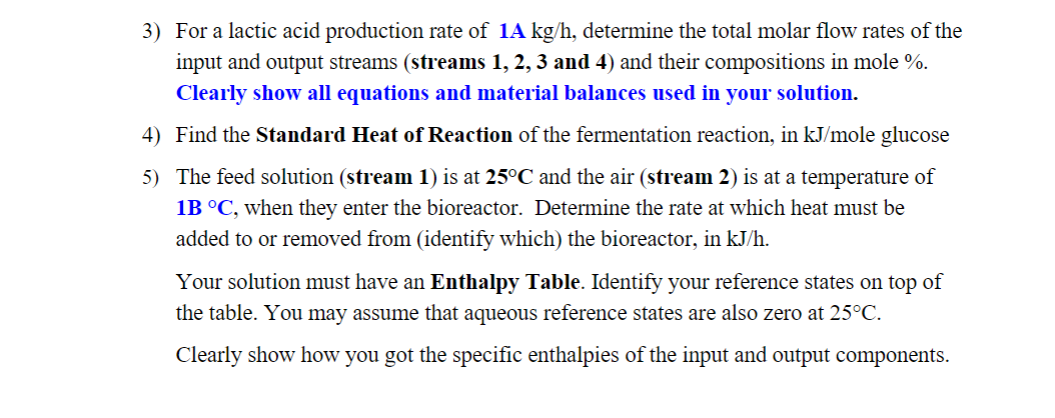
Biodegradable polymers have found increasing use in the medical field because they have the advantage of harmlessly breaking down in the body after they have served their function. Sample applications for biodegradable polymers are surgical sutures and implants that are excreted or resorbed without surgical removal. Most biodegradable substances are polymers of organic acids, and a common raw material is lactic acid. Lactic acid (C3H6O3) can be produced by fermentation using the fungus Rhizopus in liquid media, under tightly controlled bioreactor operating conditions. The process involves growth of the microorganism in a fermenter with the addition of glucose, nutrients, water, and air that is bubbled through the fermentation broth. As part of the evaluation of a proposed continuous process, you have been asked to estimate the heating or cooling requirement of a bioreactor that is to produce 1Akg of lactic acid per hour. Feed to the bioreactor includes a solution (stream 1) that is 2.10 mole \% glucose (C6H12O6), and 79%N2, that provides a molar flow rate of O2 twice that of the glucose in stream 1 . Leaving the bioreactor are a gas stream (stream 3) containing N2, unreacted O2, the CO2 formed by fermentation, and water vapor. The water vapor makes-up 3 mole \% of stream 3. Also leaving the bioreactor is a liquid stream (stream 4) containing fungal cells produced in the fermenter (formula of CH1.8O0.7N0.4 ), water, lactic acid, and unreacted ammonia and glucose. All output streams are at 25C. The stoichiometry of the fermentation reaction is given by: C6H12O6(aq)+1.158NH3(aq)+1.092O2(g)0.864C3H6O3(aq)+2.895CH1.8O0.7N0.4(s)+0.513CO2(g)+2.54H2O(l) The following table gives data for selected chemical species involved in the process. Information on other species may be found in Table B.1 and B.2 (Felder) on Canvas. The process is to be operated at Steady-state. 1) Choose a Basis. Draw and fully label a flowchart of the process based on this Basis. 2) The system is designed for a 65% conversion of the limiting reactant. Do a degreeof-freedom analysis of the overall system (for material balances only). (Hint: Also consider the balance on a chemical species that does not react.) ME 200 Conservation Principles in BME Spring 2023 3) For a lactic acid production rate of 1Akg/h, determine the total molar flow rates of the input and output streams (streams 1, 2, 3 and 4) and their compositions in mole \%. Clearly show all equations and material balances used in your solution. 3) For a lactic acid production rate of 1Akg/h, determine the total molar flow rates of the input and output streams (streams 1, 2, 3 and 4) and their compositions in mole %. Clearly show all equations and material balances used in your solution. 4) Find the Standard Heat of Reaction of the fermentation reaction, in kJ/mole glucose 5) The feed solution (stream 1) is at 25C and the air (stream 2) is at a temperature of 1BC, when they enter the bioreactor. Determine the rate at which heat must be added to or removed from (identify which) the bioreactor, in kJ/h. Your solution must have an Enthalpy Table. Identify your reference states on top of the table. You may assume that aqueous reference states are also zero at 25C. Clearly show how you got the specific enthalpies of the input and output componentsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


