Question
Above is a concentration cell for measuring Ksp for silver bromide. Given that the voltage for this cell at 298K is E=0.739 V, and

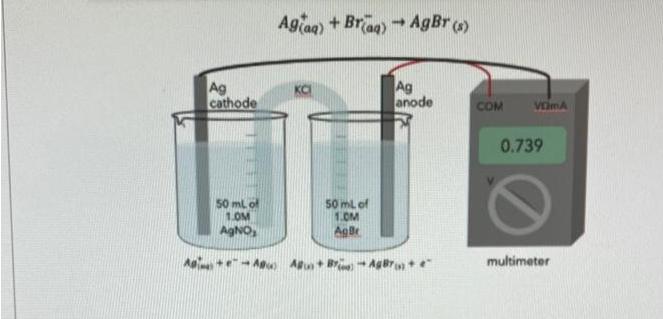
Above is a concentration cell for measuring Ksp for silver bromide. Given that the voltage for this cell at 298K is E=0.739 V, and that the overall reaction in this cell concentration cell is Use the data above to calculate pKsp, at equilibrium where pKsp = -log(Ksp) (note that when set-up with a voltmeter like this no significant current is drawn and the system reaches equilibrium) Ag cathode 50 mL of 1.0M AgNO, Agiaq) + Braq) = AgBr (s) + Ag anode 50 mL of 1.0M AgBr A+A) A+B-AgBr+ e COM VEMA 0.739 multimeter
Step by Step Solution
3.41 Rating (145 Votes )
There are 3 Steps involved in it
Step: 1
To calculate pKs we must first identify the reaction that occurs in the concentration cell The volta...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



