Above is the information
 plz do the 4a part based on the information
plz do the 4a part based on the information
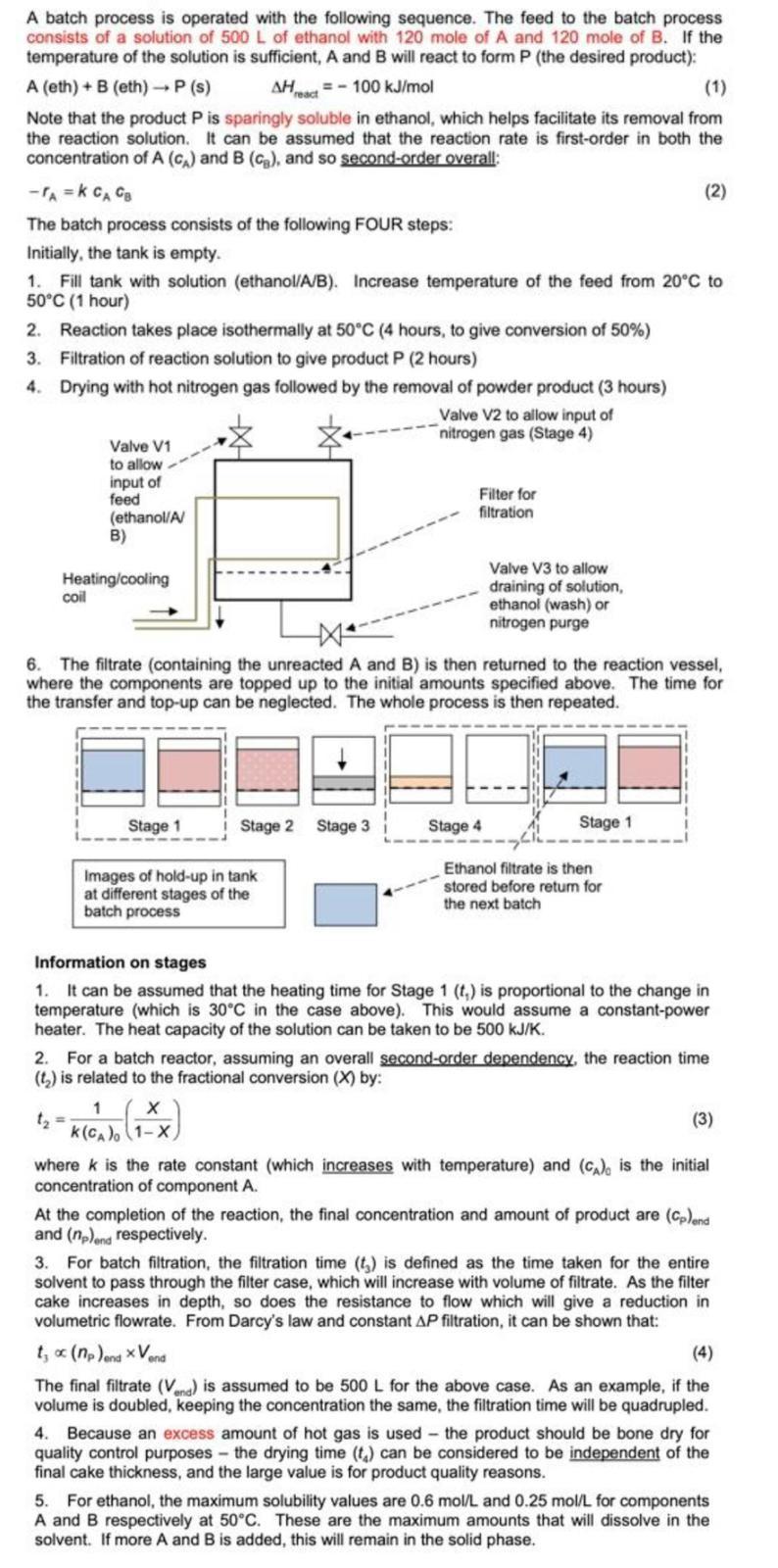
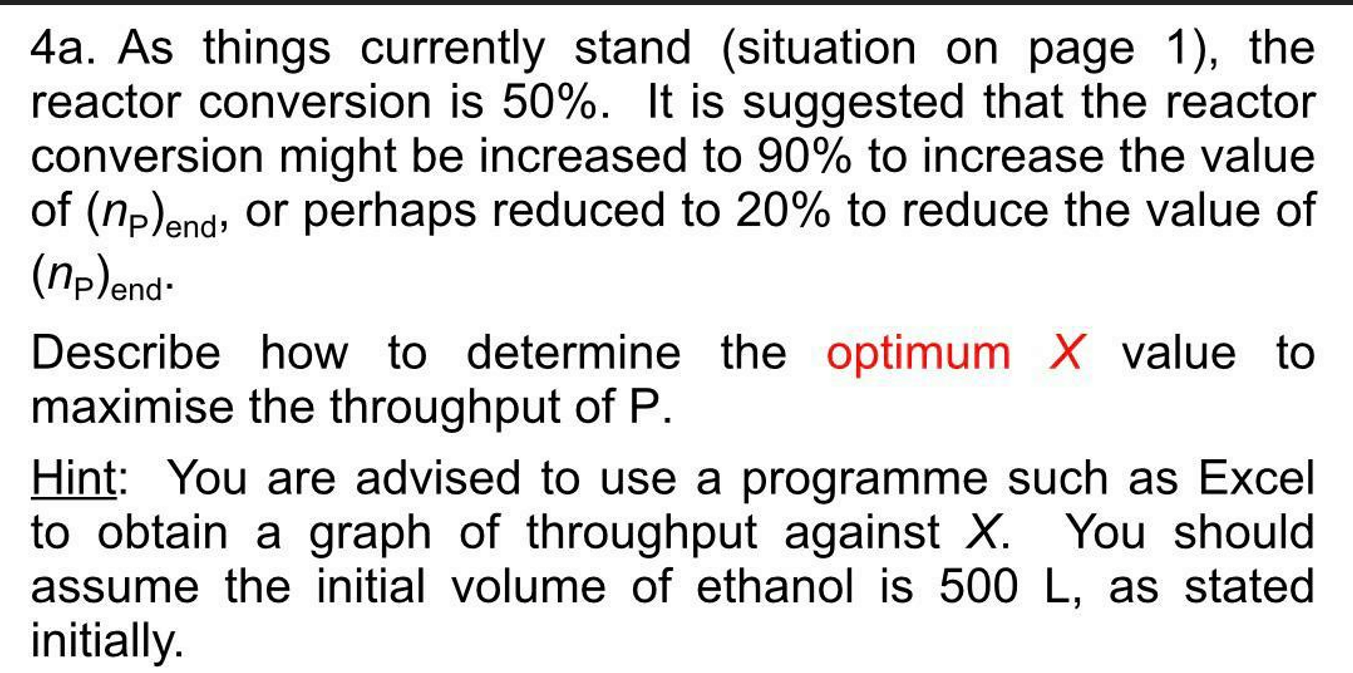
A batch process is operated with the following sequence. The feed to the batch process consists of a solution of 500 L of ethanol with 120 mole of A and 120 mole of B. If the temperature of the solution is sufficient, A and B will react to form P (the desired product): A (eth) + B (eth) - P(s) AH = - 100 kJ/ mol (1) Note that the product Pis sparingly soluble in ethanol, which helps facilitate its removal from the reaction solution. It can be assumed that the reaction rate is first-order in both the concentration of A (c) and B (og), and so second-order overall: -A=k CA Cg (2) The batch process consists of the following FOUR steps: Initially, the tank is empty. 1. Fill tank with solution (ethanol/A/B). Increase temperature of the feed from 20C to 50C (1 hour) 2. Reaction takes place isothermally at 50C (4 hours, to give conversion of 50%) 3. Filtration of reaction solution to give product P (2 hours) 4. Drying with hot nitrogen gas followed by the removal of powder product (3 hours) Valve V2 to allow input of nitrogen gas (Stage 4) Valve V1 to allow input of feed Filter for (ethanol/A filtration B) Heating/cooling coil Valve V3 to allow draining of solution, ethanol (wash) or nitrogen purge 6. The filtrate (containing the unreacted A and B) is then returned to the reaction vessel, where the components are topped up to the initial amounts specified above. The time for the transfer and top-up can be neglected. The whole process is then repeated. Stage 1 Stage 2 Stage 3 Stage 4 Stage 1 Images of hold-up in tank at different stages of the batch process Ethanol filtrate is then stored before retum for the next batch Information on stages 1. It can be assumed that the heating time for Stage 1 (1) is proportional to the change in temperature (which is 30C in the case above). This would assume a constant-power heater. The heat capacity of the solution can be taken to be 500 kJ/K. 2. For a batch reactor, assuming an overall second-order dependency, the reaction time (1) is related to the fractional conversion (X) by: 1 (3) K(CA), (1-X where k is the rate constant (which increases with temperature) and (CA), is the initial concentration of component A. At the completion of the reaction, the final concentration and amount of product are (cp)end and (np)and respectively. 3. For batch filtration, the filtration time (t) is defined as the time taken for the entire solvent to pass through the filter case, which will increase with volume of filtrate. As the filter cake increases in depth, so does the resistance to flow which will give a reduction in volumetric flowrate. From Darcy's law and constant AP filtration, it can be shown that: t; (np)ena Vond (4) The final filtrate (Vend) is assumed to be 500 L for the above case. As an example, if the volume is doubled, keeping the concentration the same, the filtration time will be quadrupled. 4. Because an excess amount of hot gas is used the product should be bone dry for quality control purposes - the drying time (t) can be considered to be independent of the final cake thickness, and the large value is for product quality reasons. 5. For ethanol, the maximum solubility values are 0.6 mol/L and 0.25 mol/L for components A and B respectively at 50C. These are the maximum amounts that will dissolve in the solvent. If more A and B is added, this will remain in the solid phase. 4a. As things currently stand (situation on page 1), the reactor conversion is 50%. It is suggested that the reactor conversion might be increased to 90% to increase the value of (np)end, or perhaps reduced to 20% to reduce the value of (np)end Describe how to determine the optimum X value to maximise the throughput of P. Hint: You are advised to use a programme such as Excel to obtain a graph of throughput against X. You should assume the initial volume of ethanol is 500 L, as stated initially A batch process is operated with the following sequence. The feed to the batch process consists of a solution of 500 L of ethanol with 120 mole of A and 120 mole of B. If the temperature of the solution is sufficient, A and B will react to form P (the desired product): A (eth) + B (eth) - P(s) AH = - 100 kJ/ mol (1) Note that the product Pis sparingly soluble in ethanol, which helps facilitate its removal from the reaction solution. It can be assumed that the reaction rate is first-order in both the concentration of A (c) and B (og), and so second-order overall: -A=k CA Cg (2) The batch process consists of the following FOUR steps: Initially, the tank is empty. 1. Fill tank with solution (ethanol/A/B). Increase temperature of the feed from 20C to 50C (1 hour) 2. Reaction takes place isothermally at 50C (4 hours, to give conversion of 50%) 3. Filtration of reaction solution to give product P (2 hours) 4. Drying with hot nitrogen gas followed by the removal of powder product (3 hours) Valve V2 to allow input of nitrogen gas (Stage 4) Valve V1 to allow input of feed Filter for (ethanol/A filtration B) Heating/cooling coil Valve V3 to allow draining of solution, ethanol (wash) or nitrogen purge 6. The filtrate (containing the unreacted A and B) is then returned to the reaction vessel, where the components are topped up to the initial amounts specified above. The time for the transfer and top-up can be neglected. The whole process is then repeated. Stage 1 Stage 2 Stage 3 Stage 4 Stage 1 Images of hold-up in tank at different stages of the batch process Ethanol filtrate is then stored before retum for the next batch Information on stages 1. It can be assumed that the heating time for Stage 1 (1) is proportional to the change in temperature (which is 30C in the case above). This would assume a constant-power heater. The heat capacity of the solution can be taken to be 500 kJ/K. 2. For a batch reactor, assuming an overall second-order dependency, the reaction time (1) is related to the fractional conversion (X) by: 1 (3) K(CA), (1-X where k is the rate constant (which increases with temperature) and (CA), is the initial concentration of component A. At the completion of the reaction, the final concentration and amount of product are (cp)end and (np)and respectively. 3. For batch filtration, the filtration time (t) is defined as the time taken for the entire solvent to pass through the filter case, which will increase with volume of filtrate. As the filter cake increases in depth, so does the resistance to flow which will give a reduction in volumetric flowrate. From Darcy's law and constant AP filtration, it can be shown that: t; (np)ena Vond (4) The final filtrate (Vend) is assumed to be 500 L for the above case. As an example, if the volume is doubled, keeping the concentration the same, the filtration time will be quadrupled. 4. Because an excess amount of hot gas is used the product should be bone dry for quality control purposes - the drying time (t) can be considered to be independent of the final cake thickness, and the large value is for product quality reasons. 5. For ethanol, the maximum solubility values are 0.6 mol/L and 0.25 mol/L for components A and B respectively at 50C. These are the maximum amounts that will dissolve in the solvent. If more A and B is added, this will remain in the solid phase. 4a. As things currently stand (situation on page 1), the reactor conversion is 50%. It is suggested that the reactor conversion might be increased to 90% to increase the value of (np)end, or perhaps reduced to 20% to reduce the value of (np)end Describe how to determine the optimum X value to maximise the throughput of P. Hint: You are advised to use a programme such as Excel to obtain a graph of throughput against X. You should assume the initial volume of ethanol is 500 L, as stated initially

 plz do the 4a part based on the information
plz do the 4a part based on the information





