Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Above what temperature will -cristobalite become the stable form of SiO 2 (i.e., more stable than -quartz) at 1 bar? Formula Form Mol, wt. g
Above what temperature will -cristobalite become the stable form of SiO2 (i.e., more stable than -quartz) at 1 bar? 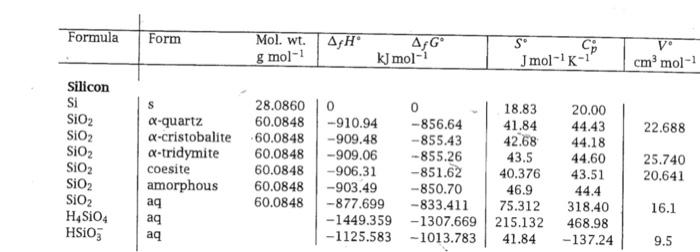
Formula Form Mol, wt. g mol-1 AfH AG kJ mol- S Jmol-'K- --k- v cm mol- 22.688 Silicon Si SIO2 SiO2 SiO2 SIO2 SIO2 SIO2 H4S104 HSO3 28.0860 a-quartz 60.0848 a-cristobalite 60.0848 a-tridymite 60.0848 coesite 60.0848 amorphous 60.0848 aq 60.0848 ag aq 0 0 18.83 -910.94 -856.64 41.84 -909.48 --855.43 42.68 -909.06 --855.26 43.5 -906.31 -851.62 40.376 -903.49 -850.70 46.9 -877.699 -833.411 75.312 -1449.359-1307.669 215.132 -1125.583-1013.783 41.84 20.00 44.43 44.18 44.60 43.51 44.4 318.40 468.98 - 137.24 25.740 20.641 16.1 9.5 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


