Answered step by step
Verified Expert Solution
Question
1 Approved Answer
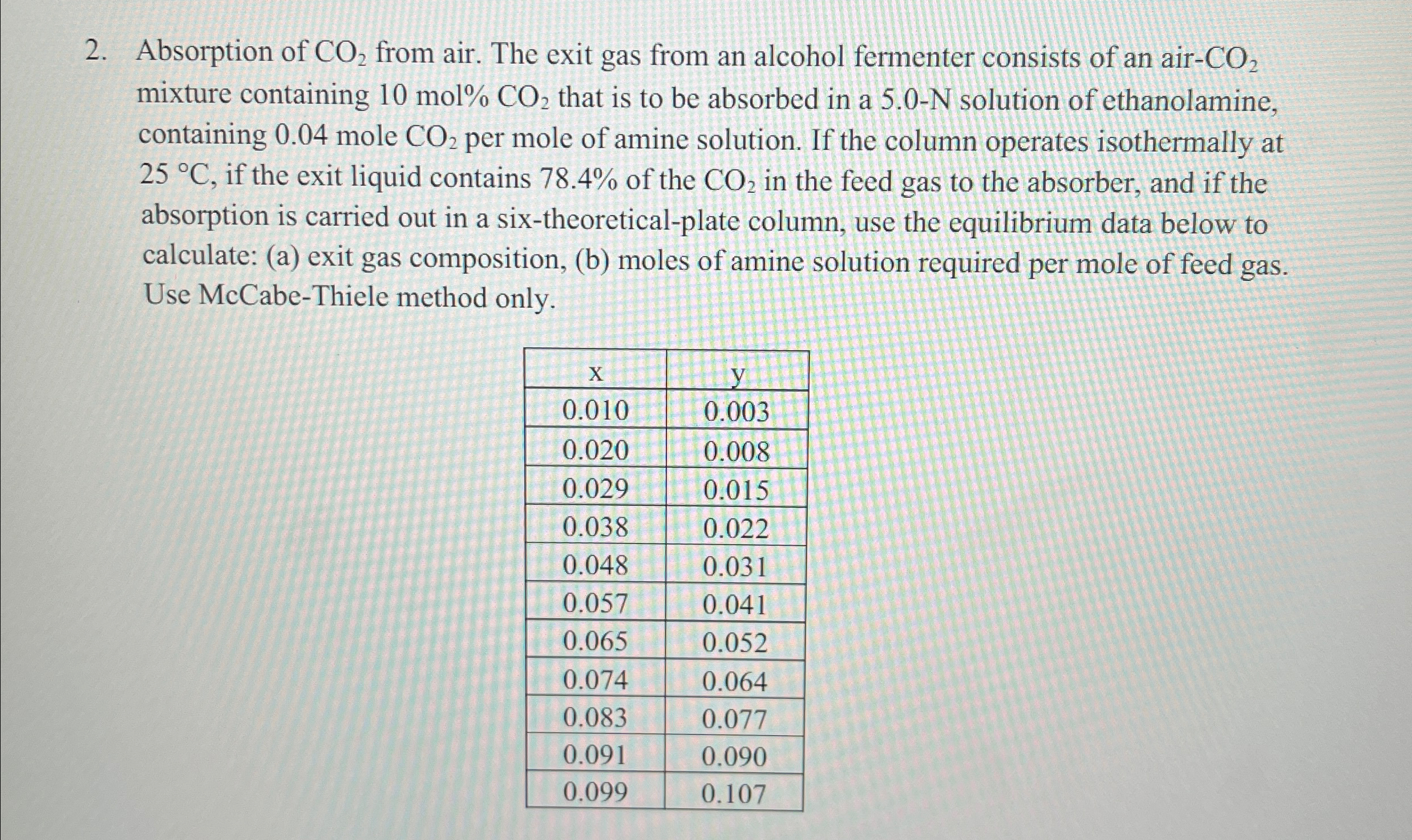
Absorption of C O 2 from air. The exit gas from an alcohol fermenter consists of an air - C O 2 mixture containing 1
Absorption of from air. The exit gas from an alcohol fermenter consists of an air mixture containing mol that is to be absorbed in a solution of ethanolamine, containing mole per mole of amine solution. If the column operates isothermally at if the exit liquid contains of the in the feed gas to the absorber and if the absorption is carried out in a sixtheoreticalplate column, use the equilibrium data below to calculate: a exit gas composition, b moles of amine solution required per mole of feed gas. Use McCabeThiele method only.
table
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


