Question
According to the Bohr model, can an electron in a lithium atom (Z=3) have an energy of -8.3 eV? Why or why not? (3
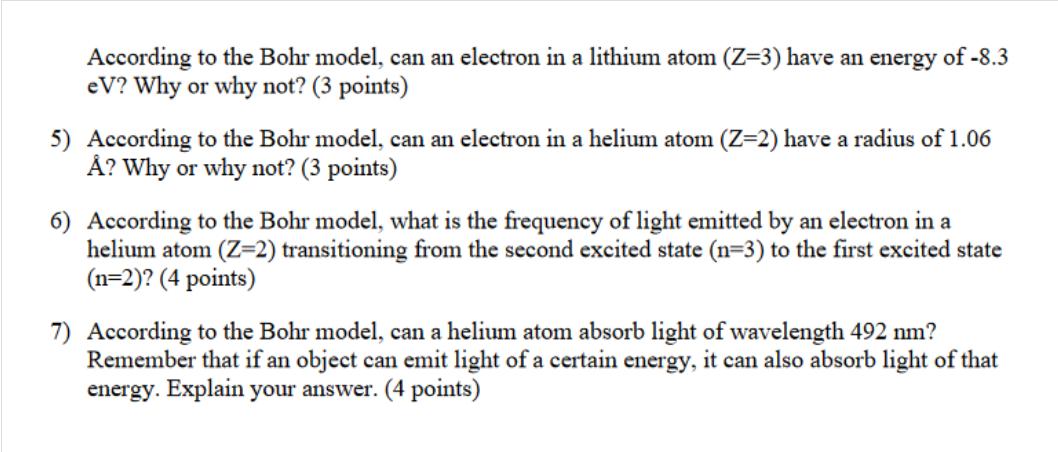
According to the Bohr model, can an electron in a lithium atom (Z=3) have an energy of -8.3 eV? Why or why not? (3 points) 5) According to the Bohr model, can an electron in a helium atom (Z=2) have a radius of 1.06 ? Why or why not? (3 points) 6) According to the Bohr model, what is the frequency of light emitted by an electron in a helium atom (Z=2) transitioning from the second excited state (n=3) to the first excited state (n=2)? (4 points) 7) According to the Bohr model, can a helium atom absorb light of wavelength 492 nm? Remember that if an object can emit light of a certain energy, it can also absorb light of that energy. Explain your answer. (4 points)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: David Young, Shane Stadler
10th edition
1118486897, 978-1118836873, 1118836871, 978-1118899205, 1118899202, 978-1118486894
Students also viewed these Algorithms questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



