Answered step by step
Verified Expert Solution
Question
1 Approved Answer
According to the Equipartition of Energy principle, the average kinetic energy of each degree of freedom in a system is considered as equal once the
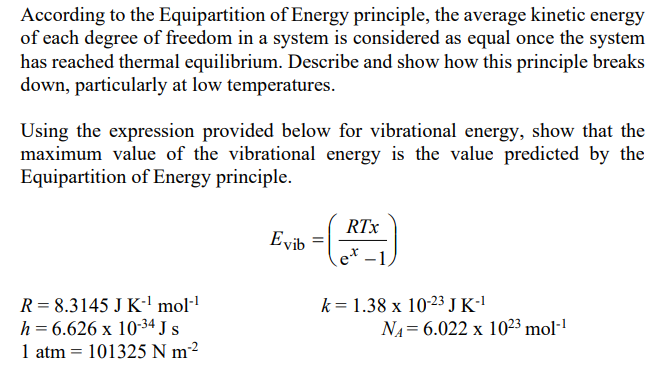
According to the Equipartition of Energy principle, the average kinetic energy of each degree of freedom in a system is considered as equal once the system has reached thermal equilibrium. Describe and show how this principle breaks down, particularly at low temperatures. Using the expression provided below for vibrational energy, show that the maximum value of the vibrational energy is the value predicted by the Equipartition of Energy principle.
According to the Equipartition of Energy principle, the average kinetic energy of each degree of freedom in a system is considered as equal once the system has reached thermal equilibrium. Describe and show how this principle breaks down, particularly at low temperatures. Using the expression provided below for vibrational energy, show that the maximum value of the vibrational energy is the value predicted by the Equipartition of Energy principle. RTX Evib = et-1 R = 8.3145 J K mol"? h = 6.626 x 10-34 Js 1 atm 101325 N m2 k= 1.38 x 10-23 J K-1 NA= 6.022 x 1023 mollStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


