Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. Construct a computer generated diagram illustrating the preparation of the Fe2+ stock, substock and external calibration standard solutions. Include the volumes pipetted, volumetric

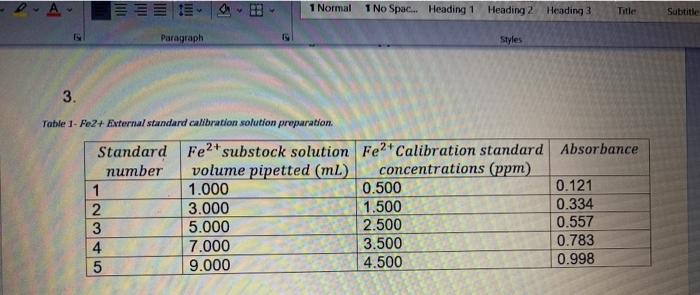

4. Construct a computer generated diagram illustrating the preparation of the Fe2+ stock, substock and external calibration standard solutions. Include the volumes pipetted, volumetric flask sizes, reagents added and actual (calculated) concentrations of all solutions. 19 1 3. Table 1- Fe2+ External standard calibration solution preparation. Standard Fe2+ substock solution volume pipetted (ml) number 1.000 23 Paragraph 4 5 3.000 5.000 1 Normal 1 No Spac... Heading 1 Heading 2 Heading 3 7.000 9.000 0.500 1.500 2.500 Styles Fe2+ Calibration standard Absorbance concentrations (ppm) 3.500 4.500 Title 0.121 0.334 0.557 0.783 0.998 Subtitle 20. Suppose that when you pipetted standard # 2 solution, you unknowingly pipetted 2.000 mL into 25.00 mL instead of 3.000 mL. When performing the calculation for the Fe concentration for this standard, you used the correct volume- 3.000 mL. (6) a) Explain how this error would affect the calibration equation and the correlation coefficient assuming that you used all five standards that you prepared. b) Explain how this mistake would affect the accuracy and precision of your final result obtained with this calibration plot. c) Explain two changes that could be made in order to accurately determine the iron concentration in the unknown samples.
Step by Step Solution
★★★★★
3.50 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


