Question
Consider the following cell written in standard notation: Pt, H(1 atm)/HCI (1 M, aq) // 1 M KCI (aq) / Ag, AgCl AGP /
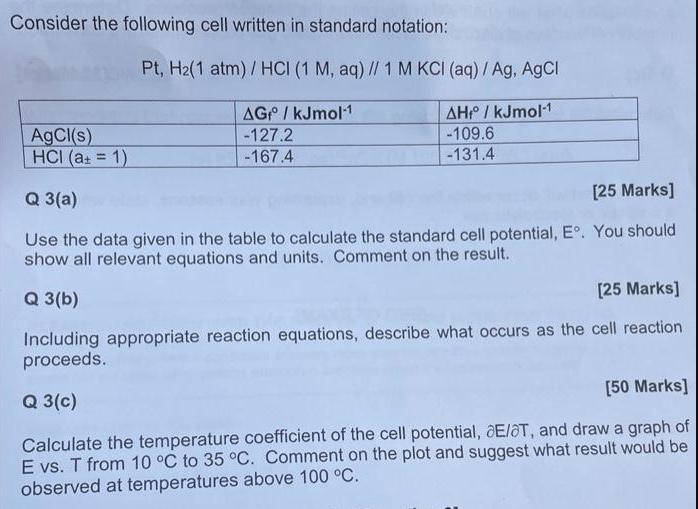
Consider the following cell written in standard notation: Pt, H(1 atm)/HCI (1 M, aq) // 1 M KCI (aq) / Ag, AgCl AGP / kJmol-1 AH / kJmol-1 -127.2 -109.6 -167.4 -131.4 AgCl(s) HCI (a = 1) Q 3(a) [25 Marks] Use the data given in the table to calculate the standard cell potential, E. You should show all relevant equations and units. Comment on the result. Q 3(b) [25 Marks] Including appropriate reaction equations, describe what occurs as the cell reaction proceeds. [50 Marks] Q 3(c) Calculate the temperature coefficient of the cell potential, E/OT, and draw a graph of E vs. T from 10 C to 35 C. Comment on the plot and suggest what result would be observed at temperatures above 100 C.
Step by Step Solution
3.44 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial Accounting Tools for business decision making
Authors: Paul D. Kimmel, Jerry J. Weygandt, Donald E. Kieso
6th Edition
978-1119191674, 047053477X, 111919167X, 978-0470534779
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



