Question
To calculate your Average value of R: Make sure you've calculated number of moles of H correctly Make sure you know Volumes of H
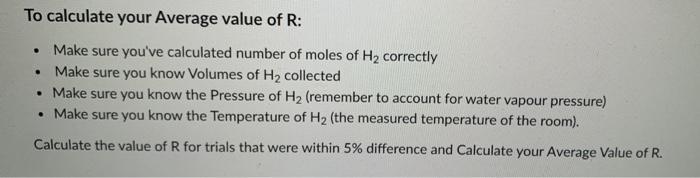
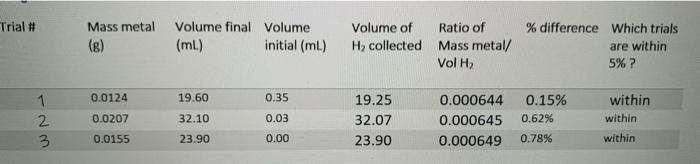
To calculate your Average value of R: Make sure you've calculated number of moles of H correctly Make sure you know Volumes of H collected Make sure you know the Pressure of H (remember to account for water vapour pressure) Make sure you know the Temperature of H (the measured temperature of the room). Calculate the value of R for trials that were within 5% difference and Calculate your Average Value of R. . . Trial # 1 wN. 2 3 Mass metal (g) 0.0124 0.0207 0.0155 Volume final Volume (ml) 19.60 32.10 23.90 initial (ml) 0.35 0.03 0.00 Volume of H collected 19.25 32.07 23.90 Ratio of Mass metal/ Vol H 0.000644 0.000645 0.000649 % difference Which trials are within 5% ? 0.15% 0.62% 0.78% within within within
Step by Step Solution
3.38 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



