Question
Acetic acid and water react to form hydronium cation and acetate anion, like this: HCH3CO2(aq)+H2O(l)H3O+(aq)+CH3CO2(aq) Imagine 62.mmol of HCH3CO2 are removed from a flask containing
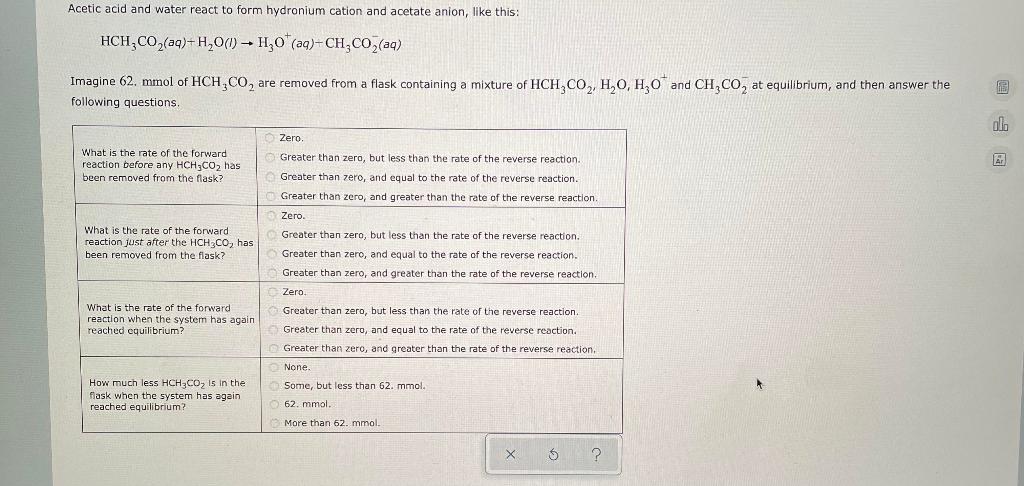 Acetic acid and water react to form hydronium cation and acetate anion, like this: HCH3CO2(aq)+H2O(l)H3O+(aq)+CH3CO2(aq) Imagine 62.mmol of HCH3CO2 are removed from a flask containing a mixture of HCH3CO2, H2O, H3O+ and CH3CO2 at equilibrium, and then answer the following questions. What is the rate of the forward reaction before any HCH3CO2 has been removed from the flask? Zero. Greater than zero, but less than the rate of the reverse reaction. Greater than zero, and equal to the rate of the reverse reaction. Greater than zero, and greater than the rate of the reverse reaction. What is the rate of the forward reaction just after the HCH3CO2 has been removed from the flask? Zero. Greater than zero, but less than the rate of the reverse reaction. Greater than zero, and equal to the rate of the reverse reaction. Greater than zero, and greater than the rate of the reverse reaction. What is the rate of the forward reaction when the system has again reached equilibrium? Zero. Greater than zero, but less than the rate of the reverse reaction. Greater than zero, and equal to the rate of the reverse reaction. Greater than zero, and greater than the rate of the reverse reaction. How much less HCH3CO2 is in the flask when the system has again reached equilibrium? None. Some, but less than 62. mmol. 62. mmol. More than 62. mmol.
Acetic acid and water react to form hydronium cation and acetate anion, like this: HCH3CO2(aq)+H2O(l)H3O+(aq)+CH3CO2(aq) Imagine 62.mmol of HCH3CO2 are removed from a flask containing a mixture of HCH3CO2, H2O, H3O+ and CH3CO2 at equilibrium, and then answer the following questions. What is the rate of the forward reaction before any HCH3CO2 has been removed from the flask? Zero. Greater than zero, but less than the rate of the reverse reaction. Greater than zero, and equal to the rate of the reverse reaction. Greater than zero, and greater than the rate of the reverse reaction. What is the rate of the forward reaction just after the HCH3CO2 has been removed from the flask? Zero. Greater than zero, but less than the rate of the reverse reaction. Greater than zero, and equal to the rate of the reverse reaction. Greater than zero, and greater than the rate of the reverse reaction. What is the rate of the forward reaction when the system has again reached equilibrium? Zero. Greater than zero, but less than the rate of the reverse reaction. Greater than zero, and equal to the rate of the reverse reaction. Greater than zero, and greater than the rate of the reverse reaction. How much less HCH3CO2 is in the flask when the system has again reached equilibrium? None. Some, but less than 62. mmol. 62. mmol. More than 62. mmol.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


