Question
Acetylene burns in air according to the following equation: CH(g) + O(g) 2C0(g) + HO(g) kJ Given AH of CO(g)=-394.4 - and AH of
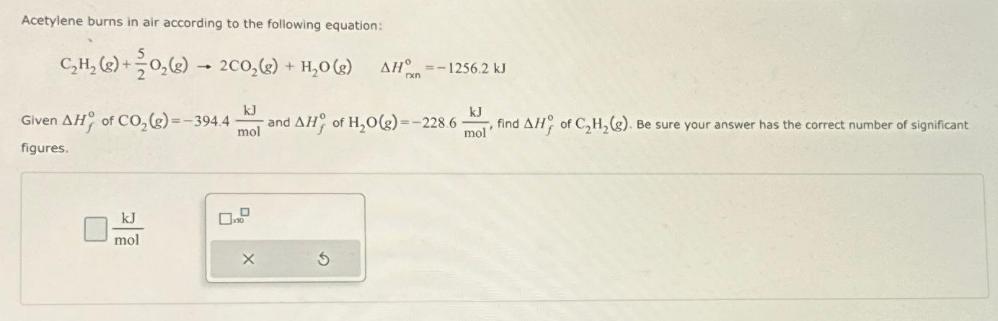
Acetylene burns in air according to the following equation: CH(g) + O(g) 2C0(g) + HO(g) kJ Given AH of CO(g)=-394.4 - and AH of HO(g)=-228.6 mol figures. kJ mol X AH-1256.2 kJ mol find AH of CH(g). Be sure your answer has the correct number of significant
Step by Step Solution
3.41 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
The question presents a chemical equation for the combustion of acetylene C2H2 and provides the enth...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry For Engineering Students
Authors: Lawrence S. Brown, Tom Holme
4th Edition
0357026993, 978-0357026991
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



