Answered step by step
Verified Expert Solution
Question
1 Approved Answer
acid base Ka K, name formula name formula 4 hydrofluoric acid HF -8 6.8 x 10 hydroxylamine HONH2 1.1 x 10 -8 hypochlorous acid HCIO
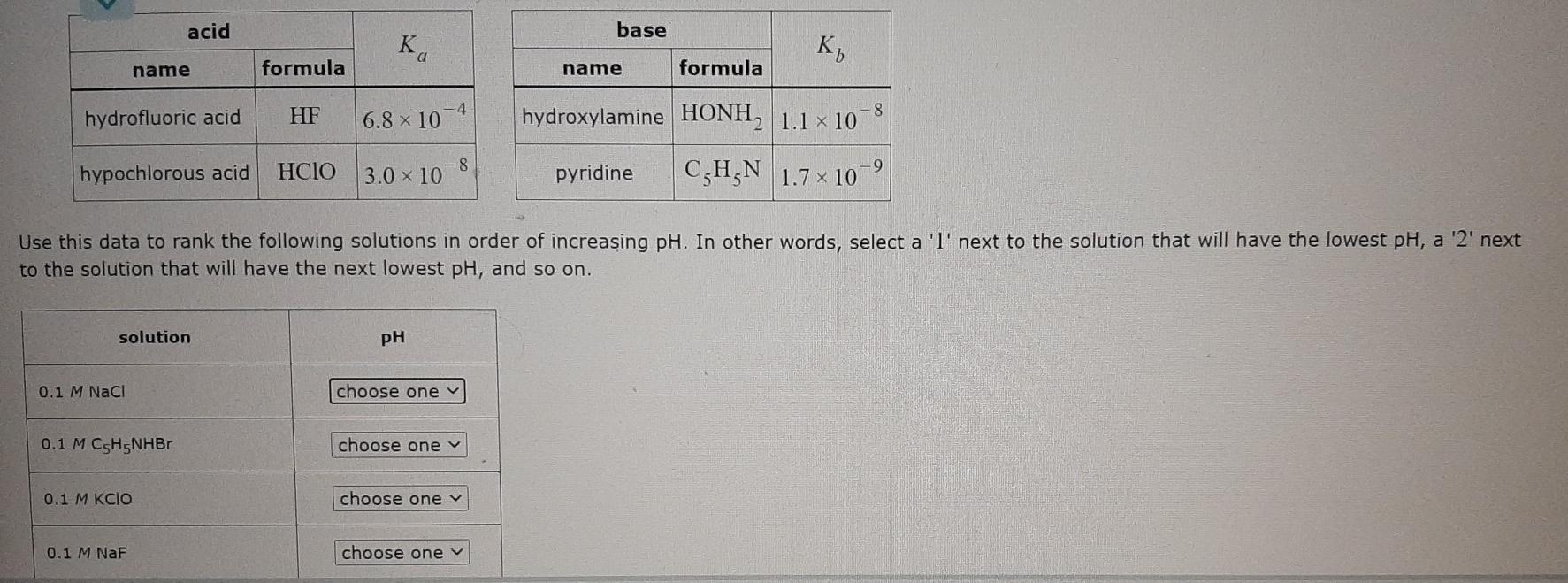
acid base Ka K, name formula name formula 4 hydrofluoric acid HF -8 6.8 x 10 hydroxylamine HONH2 1.1 x 10 -8 hypochlorous acid HCIO 3.0 x 10 pyridine C,H,N 1.7 x 10 Use this data to rank the following solutions in order of increasing pH. In other words, select a 'l' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution PH 0.1 M NaCl choose one 0.1 M CSHSNHBr choose one v 0.1 M KCIO choose one y 0.1 M NaF choose one
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


