Answered step by step
Verified Expert Solution
Question
1 Approved Answer
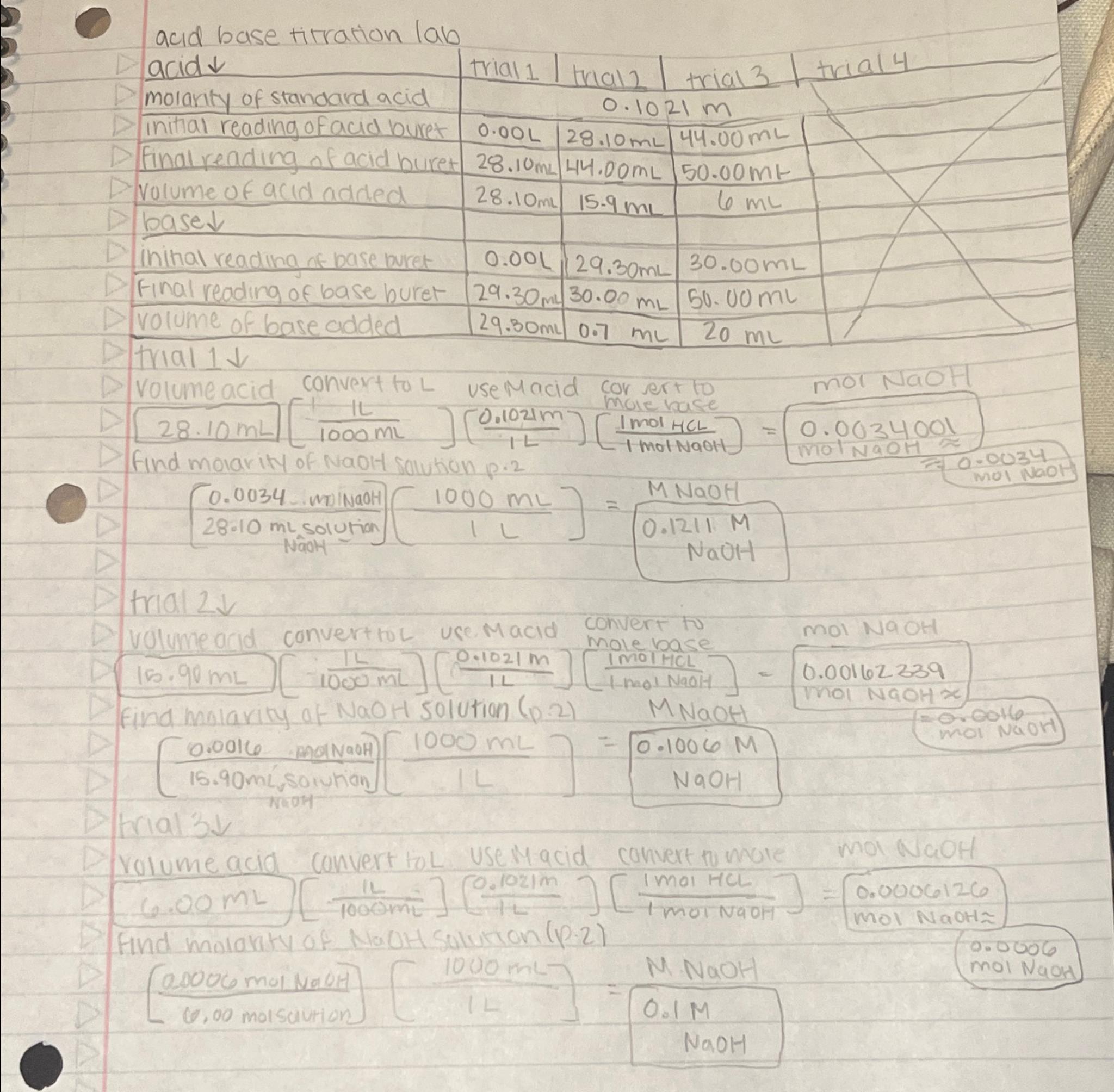
acid base titration lab table [ [ acid , trial 1 , I trial 1 , trial 3 , trial 4 ] , [
acid base titration lab
tableacidtrial I trial trial trial molarify of standard acid,Initial reading of acid buret,Final reading of acid buret,volume of acid added,base darr,,,,inithal reading of base buret,final reading of base buret,volume of base added,
trial darr
Volumeacid convert to use Macid cor ert to
find molarity of NaOH solution
trial
volumeacid convert to use Macid convert to mole base molNaOH
moi NaOH
trial
rolumeacid convert toL use Macid convert to mote mol NaOH
Find molaraty of NaOH Solution
mol NaOH
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


