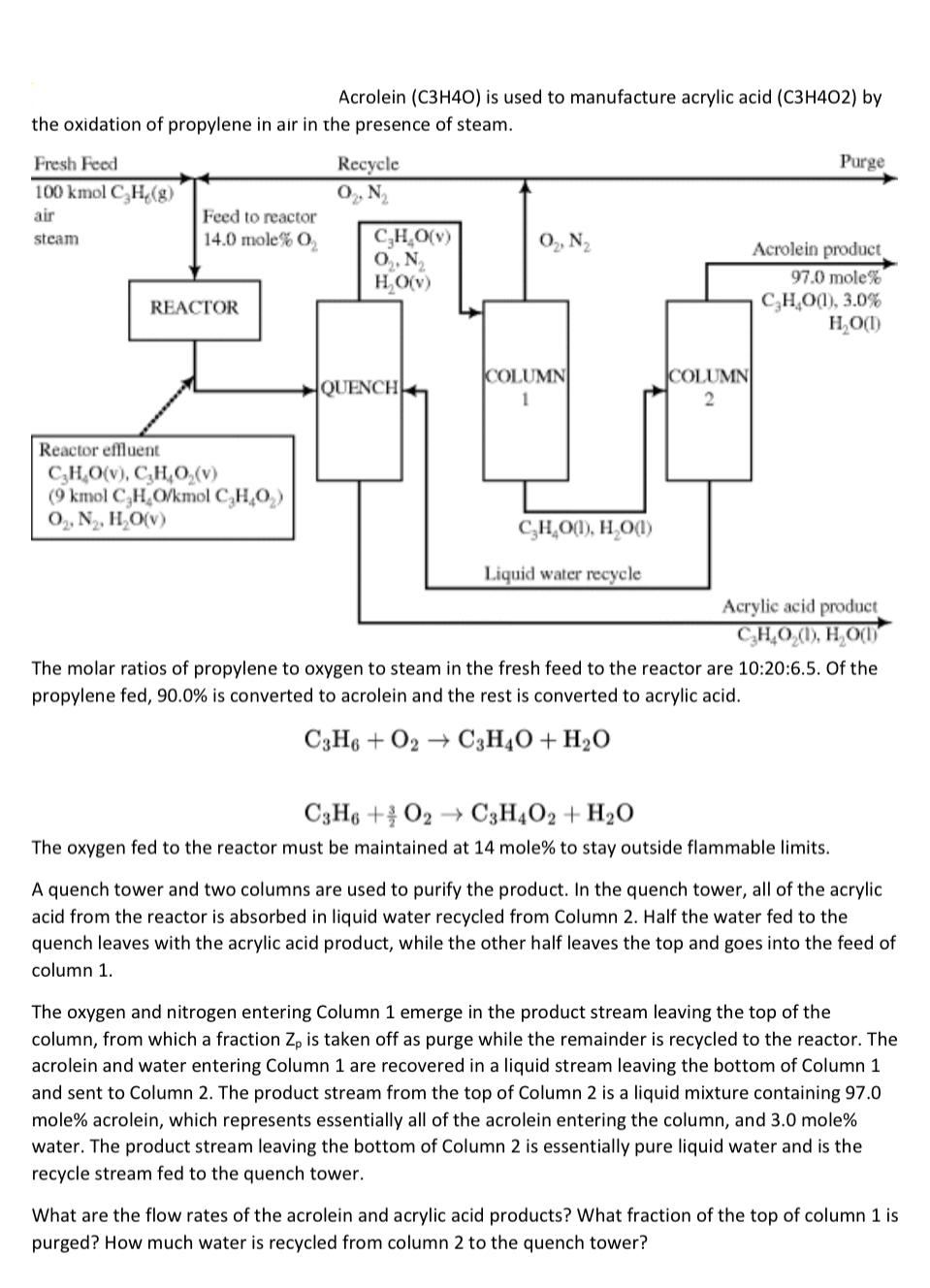
Acrolein ( C3H4O) is used to manufacture acrylic acid ( C3H4O2) by the oxidation of propylene in air in the presence of steam. The molar ratios of propylene to oxygen to steam in the fresh feed to the reactor are 10:20:6.5. Of the propylene fed, 90.0% is converted to acrolein and the rest is converted to acrylic acid. C3H6+O2C3H4O+H2OC3H6+23O2C3H4O2+H2O The oxygen fed to the reactor must be maintained at 14 mole\% to stay outside flammable limits. A quench tower and two columns are used to purify the product. In the quench tower, all of the acrylic acid from the reactor is absorbed in liquid water recycled from Column 2. Half the water fed to the quench leaves with the acrylic acid product, while the other half leaves the top and goes into the feed of column 1. The oxygen and nitrogen entering Column 1 emerge in the product stream leaving the top of the column, from which a fraction Zp is taken off as purge while the remainder is recycled to the reactor. The acrolein and water entering Column 1 are recovered in a liquid stream leaving the bottom of Column 1 and sent to Column 2. The product stream from the top of Column 2 is a liquid mixture containing 97.0 mole\% acrolein, which represents essentially all of the acrolein entering the column, and 3.0 mole\% water. The product stream leaving the bottom of Column 2 is essentially pure liquid water and is the recycle stream fed to the quench tower. What are the flow rates of the acrolein and acrylic acid products? What fraction of the top of column 1 is purged? How much water is recycled from column 2 to the quench tower? Acrolein ( C3H4O) is used to manufacture acrylic acid ( C3H4O2) by the oxidation of propylene in air in the presence of steam. The molar ratios of propylene to oxygen to steam in the fresh feed to the reactor are 10:20:6.5. Of the propylene fed, 90.0% is converted to acrolein and the rest is converted to acrylic acid. C3H6+O2C3H4O+H2OC3H6+23O2C3H4O2+H2O The oxygen fed to the reactor must be maintained at 14 mole\% to stay outside flammable limits. A quench tower and two columns are used to purify the product. In the quench tower, all of the acrylic acid from the reactor is absorbed in liquid water recycled from Column 2. Half the water fed to the quench leaves with the acrylic acid product, while the other half leaves the top and goes into the feed of column 1. The oxygen and nitrogen entering Column 1 emerge in the product stream leaving the top of the column, from which a fraction Zp is taken off as purge while the remainder is recycled to the reactor. The acrolein and water entering Column 1 are recovered in a liquid stream leaving the bottom of Column 1 and sent to Column 2. The product stream from the top of Column 2 is a liquid mixture containing 97.0 mole\% acrolein, which represents essentially all of the acrolein entering the column, and 3.0 mole\% water. The product stream leaving the bottom of Column 2 is essentially pure liquid water and is the recycle stream fed to the quench tower. What are the flow rates of the acrolein and acrylic acid products? What fraction of the top of column 1 is purged? How much water is recycled from column 2 to the quench tower







