Answered step by step
Verified Expert Solution
Question
1 Approved Answer
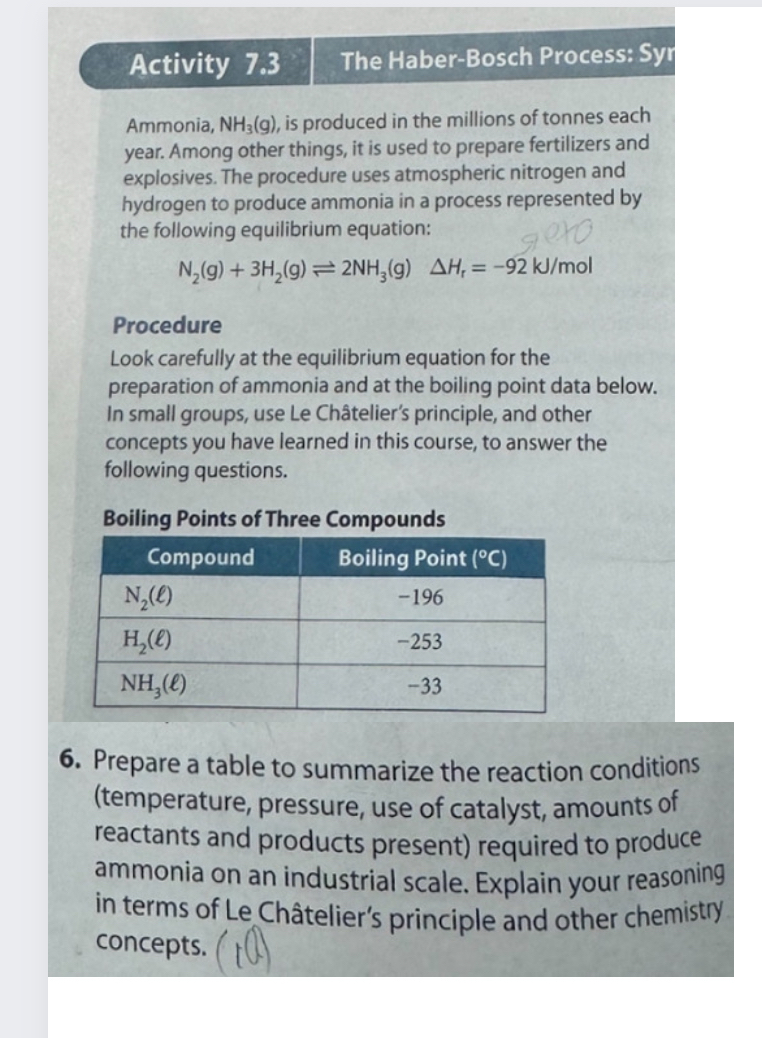
Activity 7 . 3 The Haber - Bosch Process: Syr Ammonia, N H 3 ( g ) , is produced in the millions of tonnes
Activity
The HaberBosch Process: Syr
Ammonia, is produced in the millions of tonnes each year. Among other things, it is used to prepare fertilizers and explosives. The procedure uses atmospheric nitrogen and hydrogen to produce ammonia in a process represented by the following equilibrium equation:
Procedure
Look carefully at the equilibrium equation for the preparation of ammonia and at the boiling point data below. In small groups, use Le Chteliers principle, and other concepts you have learned in this course, to answer the following questions.
Boiling Points of Three Compounds
tableCompoundBoiling Point
Prepare a table to summarize the reaction conditions temperature pressure, use of catalyst, amounts of reactants, and amount of products required to produce ammonia on an industrial scale. Explain your reasoning in terms of Le Chteliers principle and other chemistry concepts.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


