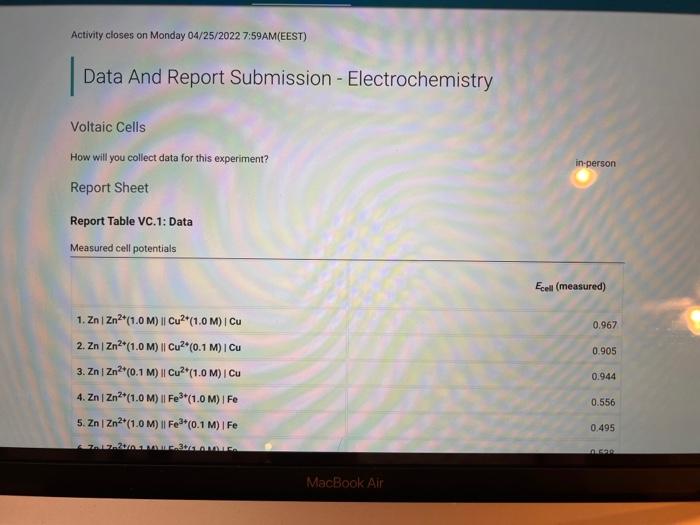
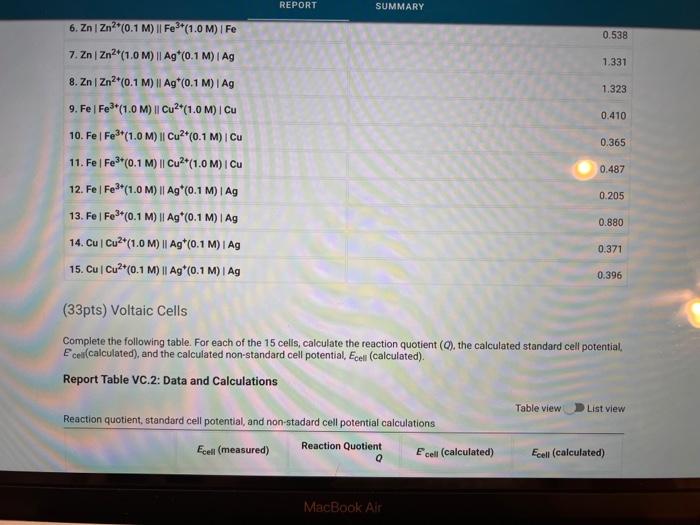
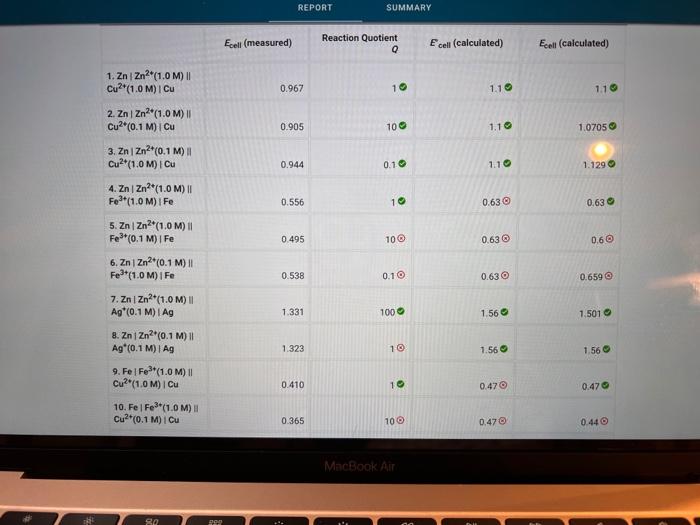
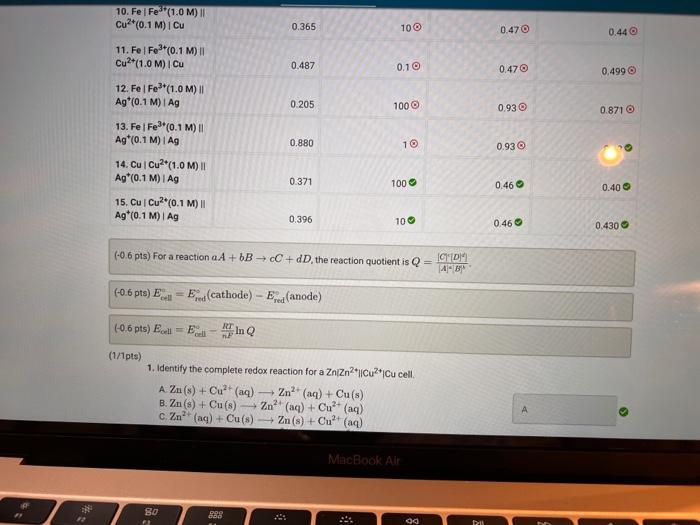
Activity closes on Monday 04/25/2022 7:59AM(EEST) 1 Data And Report Submission - Electrochemistry Voltaic Cells How will you collect data for this experiment? in-person Report Sheet Report Table VC.1: Data Measured cell potentials Ecell (measured) 0.967 0.905 1. ZnZn2+(1.0 M) || Cu2+(1.0 M) Cu 2. ZnZn2(1.0 M) || Cu2+(0.1 M) Cu 3. ZnZn2+(0.1 M) || Cu2+(1.0M) | Cu 4. Zn | Zn2(1.0M) | Fe3+(1.0M) Fe 5. ZnZn2+(1.0M) || Fe*(0.1 M)| Fe 0.944 0.556 0.495 in N MacBook Air REPORT SUMMARY 0.538 1.331 1.323 0.410 0.365 6. ZnZn?*(0.1 M) || Fe3+(1.0M) / Fe 7. ZnZn2+(1.0 M) || Ag (0.1 M) I Ag 8. ZnZn2+(0.1 M) || Ag*(0.1 M) | Ag 9. Fel Fe*(1.0 M) Cu2+(1.0 M) Cu 10. Fel Fe**(1.0M) || Cu?*(0.1 M) Cu 11. Fel Fe3+(0.1 M) || Cu2+(1.0M) | Cu 12. Fel Fe3+(1.0 M) || Ag*(0.1 M) | Ag 13. Fel Fe3+(0.1 M) || Ag*(0.1 M) I Ag 14. Cu Cu2+(1.0 M) || Ag(0.1 M) | Ag 15. Cu Cu2+(0.1 M) || Ag*(0.1 M)| Ag 0.487 0.205 0.880 0.371 0.396 (33pts) Voltaic Cells Complete the following table. For each of the 15 cells, calculate the reaction quotient (C), the calculated standard cell potential Ece (calculated), and the calculated non-standard cell potential, Ecell (calculated). Report Table VC.2: Data and Calculations Table view List view Reaction quotient, standard cell potential, and non-stadard cell potential calculations Ecell (measured) Reaction Quotient Q Ecell (calculated) Ecell (calculated) MacBook Air REPORT SUMMARY Ecell (measured) Reaction Quotient Q Ecell (calculated) Ecell (calculated) 1. ZnZn2+(1.0M) 1 Cu2(1.0 M) Cu 0.967 1 1.10 1.10 0.905 100 1.1 1.0705 0.944 0.1 1.10 1.129 0.556 10 0.63 0.63 0.495 10 0.63 0.6 2. ZnZn2+(1.0M) Cu2*(0.1 M) Cu 3. ZnZn2+(0.1 M) Cu2(1.0M) Cu 4. ZnZn2+(1.0 M) Fe2+(1.0M) IF 5. Zn | Zn2+(1.0M) 11 Fe*(0,1 M) | Fe 6. ZnZn2+(0.1 M) Fe3+(1.0M) Fe 7. ZnZn2+(1.0M) 11 Ag (0.1 M) TAG 8. ZnZn2+(0.1 M) Ag (0.1 M) | Ag 9.Fe Fer(1.0 M) Cu2+(1.0M) Cu 0.538 0.1 0.63 0.659 1.331 100 1.56 1.501 1.323 1 1.56 1.56 0.410 1 0.47 0.47 10. Fel Fe*(1.0 M) Cu2(0.1 M) Cu 0.365 100 0.47 0.44 MacBook Air 27 so 290 0.365 100 0.470 0.44 0.487 0.1 0.47 0,499 0.205 1000 0.93 10.Fe Fes"(1.0M) Cu2+(0.1 M) Cu 11. Fe Fe (0.1 M) Cu2(1.0M) Cu 12. Fel Fe*(1.0M) 11 Ag (0.1 M) I Ag 13. Fe Fe3+(0.1 M) Ag (0.1 M) Ag 14. Cu Cu2+(1.0M) 11 Ag (0.1 M)1 Ag 15. Cu Cu2*(0.1 M) 11 Ag*(0.1 M) | AG 0.8710 0.880 10 0.93 . 0.371 100 0.46 0.40 0.396 100 0.46 0.430 (-0.6 pts) For a reaction a A + B C + dDthe reaction quotient is Q [ClD]] AU (-0.6 pts) E. Erd(cathode) - Ered (anode) -0.6 pts) Ecoli = Ein Q (1/1pts) 1. Identify the complete redox reaction for a ZnZn2||Cucu cell. A Zn(s) + Cu?+ (aq) Zn2+ (aq) + Cu(s) B. Zn(s) + Cu(s) Zn2+ (aq) + Cu?+ (aq) c. Zn2+ (aq) + Cu(s) Zn(s) + Cu? (aq) MacBook Air 80 888 . 04 Data and Report Submission WOOD REPORT SUMMARY 2+ (0/1pts) 2. Identify the complete redox reaction for a ZnZnFFe cell. A 3 Zn(s) + 2 Fe(s) - 3 Zn2+ (aq) + 2Fe+ (aq) 8.3 Zn2+ (aq) + 2 Fe(s) 3 Zn (8) + 2Fe+ (aq) c Zn(s) + 3Fe (aq) -Zn+ (aq) +3 Fe(8) 0.3 Zn(s) + 2Fe+ (aq) +3Zn+ (aq) + 2 Fe (8) B (-1 pts) Incorrect + + (1/1pts) 3. Identify the complete redox reaction for a Zn/Zn?|Ag*Ag cell A Zn(s) + Ag (8) - Zn?" (aq) + Ag+ (aq) B. Zn+ (aq) + Ag(s) Zn(s) + Ag+ (aq) C. Zn(s) + 2 Ag(n)- Zn(aq) + 2 Ag(s) D. Zn(s) + Ag+ (aq) - Zn2+ (aq) + Ag(s) (1/1pts) 4. Identify the complete redox reaction for a Felfel|cucu cell, A2 Fe(s) + 3Cu(s) - 2Fe (aq) + 3 Cu?+ (aq) B. 2 Fe(s) + 3 Cu?+ (aq) + 2F+ (aq) + 3Cu(s) C. 2Fe(aq) + 3Cu() +2 Fe(s) + 3 Cu?+ (aq) D. Fe(s) + 2 Cu?() - Fe+ (aq) + 2 Cu(s) (0/1pts) 5. Identify the complete redox reaction for a Felfe*AAg cell. A Fe(s) + Ag (8) Fe? (aq) + Ag (aq) B. Fe(s) + Ag (aq) Fe+ (aq) + Ag (8) c. Fe (aq) + Ag(s) Fe(s) + Ag+ (aq) Fos) + 3 Ag(89) - Felag - 3 Aga B 11 C MacBook Air RO ROM ga DD