Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Add 2 mL volume of decarbonated soda to each test tube. Add the value of the working solution of tartrazine to each test tube. Dilute
Add 2 mL volume of decarbonated soda to each test tube. Add the value of the working solution of tartrazine to each test tube. Dilute the solution with DI water to a total of 6 mL. Calculate the concentration of tartrazine in the undiluted soda.
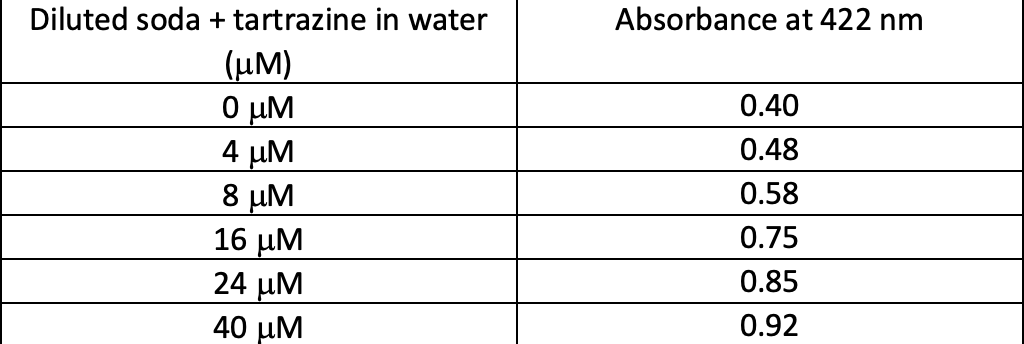
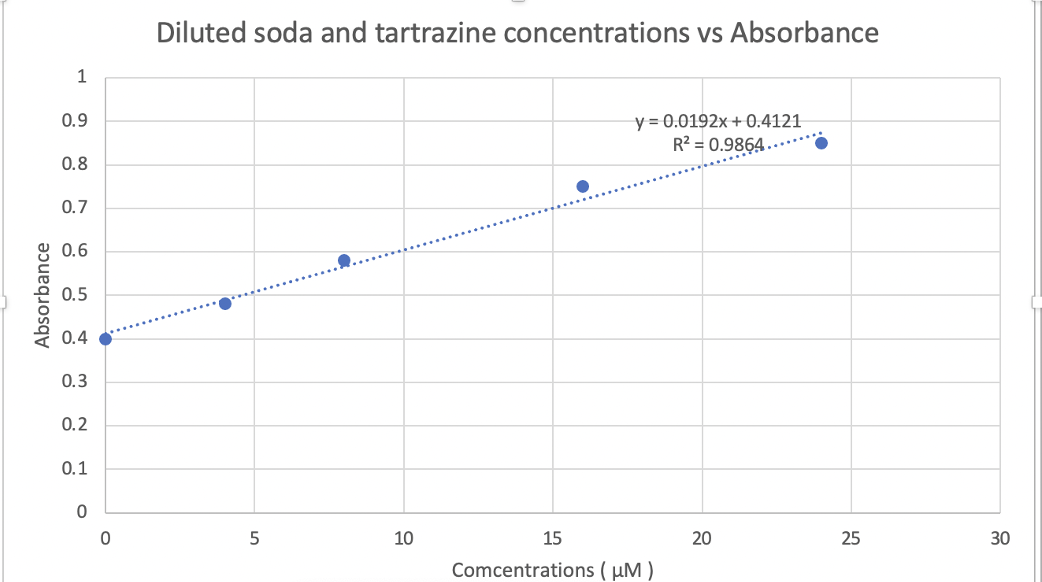
Absorbance at 422 nm Diluted soda + tartrazine in water () M 4 M 8 M 16 M 24 M 40 M 0.40 0.48 0.58 0.75 0.85 0.92 Diluted soda and tartrazine concentrations vs Absorbance 1 0.9 y = 0.0192x + 0.4121 R2 = 0.9864 0.8 0.7 0.6 Absorbance 0.5 0.4 0.3 0.2 0.1 0 o 5 10 15 20 25 30 Comcentrations (UM)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


