Question
Add sample of NH4NO3(s) to water and measured the temperature over time. The data are shown in the graph above. (a) What is the value
Add sample of NH4NO3(s) to water and measured the temperature over time. The data are shown in the graph above.
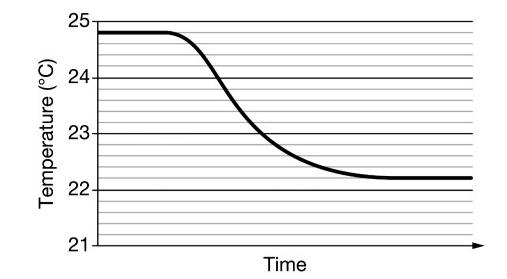
(a) What is the value of ΔT that the student should use to calculate q ?
(b) According to the graph, is the dissolution ofNH4NO3(s) endothermic or exothermic? Explain.
(c) Are the strengths of the interactions between the particles in the solute and between the particles in the solvent before the solute and solvent are combined greater than, less than, or equal to the strengths of the interactions between solute particles and solvent particles after dissolution? Explain.
25- 24- 23 22- 21 Time Temperature (C)
Step by Step Solution
3.35 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
a In order to calculate q the student should take T 24822226C b As we can see that the final t...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Graham Solomons, Craig Fryhle, Scott Snyder
11th edition
1118133579, 978-1118133576
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



