Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Additional Info: k= 10min -1 K C =0.01mol 2 /dm 6 k CA =1min -1 k CB =40min -1 F A0 =100mol/min v 0 =
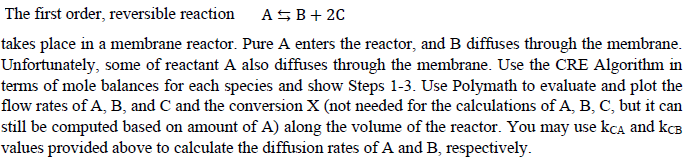
Additional Info:
k= 10min-1
KC=0.01mol2/dm6
kCA=1min-1
kCB=40min-1
FA0=100mol/min
v0 = 100dm3/min
VReactor=20dm3
The first order, reversible reaction AB+2C takes place in a membrane reactor. Pure A enters the reactor, and B diffuses through the membrane. Unfortunately, some of reactant A also diffuses through the membrane. Use the CRE Algorithm in terms of mole balances for each species and show Steps 1-3. Use Polymath to evaluate and plot the flow rates of A,B, and C and the conversion X (not needed for the calculations of A,B,C, but it can still be computed based on amount of A ) along the volume of the reactor. You may use kCA and kCB values provided above to calculate the diffusion rates of A and B, respectivelyStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


