Answered step by step
Verified Expert Solution
Question
1 Approved Answer
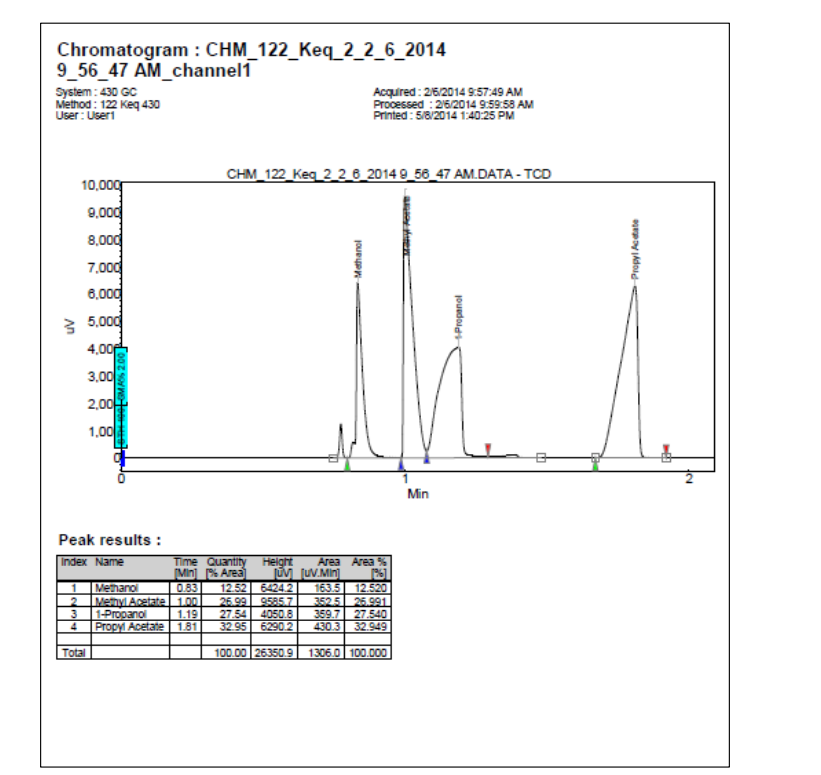
Advance Study Assignment Calculate the numerical value of the equilibrium constant for the reaction on the first page of this experiment, using the Area %
Advance Study Assignment
Calculate the numerical value of the equilibrium constant for the reaction on the
first page of this experiment, using the Area column on the Peak results
table under the third sample GC Use the column on the very far right for the
percents. Make sure your answer is in scientific notation and to the correct number
of significant digits.
This answer will not be the same as your final value calculation, since these four
chemicals were just poured together to get the sample GC and not a result of
doing this experiment. All of your work must be typed or on a computer printout.
No handwritten answers will be accepted for labs. Turn in this completed page,
with your setup and answer for stamping, with your prelab. You will get it back to
include in your final lab report after it is stamped.
K value answer
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


