Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Advanced thermodynamic 10.28 Given below are values of GE/Jmol1,HE/Jmol1, and CPE/Jmol1K1 for some equimolar binary liquid mixtures at 298.15K. Estimate values of GE,HE, and SE
Advanced thermodynamic 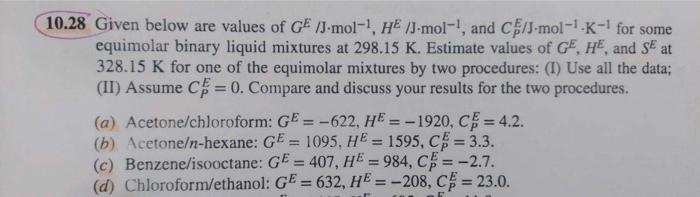
10.28 Given below are values of GE/Jmol1,HE/Jmol1, and CPE/Jmol1K1 for some equimolar binary liquid mixtures at 298.15K. Estimate values of GE,HE, and SE at 328.15K for one of the equimolar mixtures by two procedures: (I) Use all the data; (II) Assume CPE=0. Compare and discuss your results for the two procedures. (a) Acetone/chloroform: GE=622,HE=1920,CPE=4.2. (b) Acetone -hexane: GE=1095,HE=1595,CPE=3.3. (c) Benzene/isooctane: GE=407,HE=984,CPE=2.7. (d) Chloroform/ethanol: GE=632,HE=208,CPE=23.0 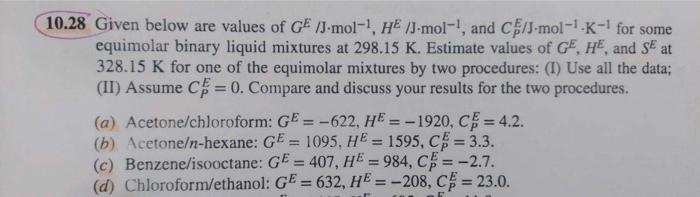
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


