Answered step by step
Verified Expert Solution
Question
1 Approved Answer
After preparing the graphs, answer these questions: Experiment 1 What is the general trend in the solubility of potassium nitrate as temperature increase the general
After preparing the graphs, answer these questions:
Experiment
What is the general trend in the solubility of potassium nitrate as temperature increase the general trend of the solubility of potassium increases as the temperature increases.
What amount of potassium nitrate would dissolve at
ahout of woold dissolve
How much potassium nitrate would dissolve in of water at
How much potassium nitrate would dissolve in of water at
Experiment
Which is the independent variable, mass or volume?
the independent variable is Volvme.
What is the general trend as mass increases, what happens to the volume? the volume also increases.
What physical quantity is described as the amount of mass divided by the substance?
denjity
magail
Nunever add timndlike, it wrill be linear.
large as possible.
titu laveled
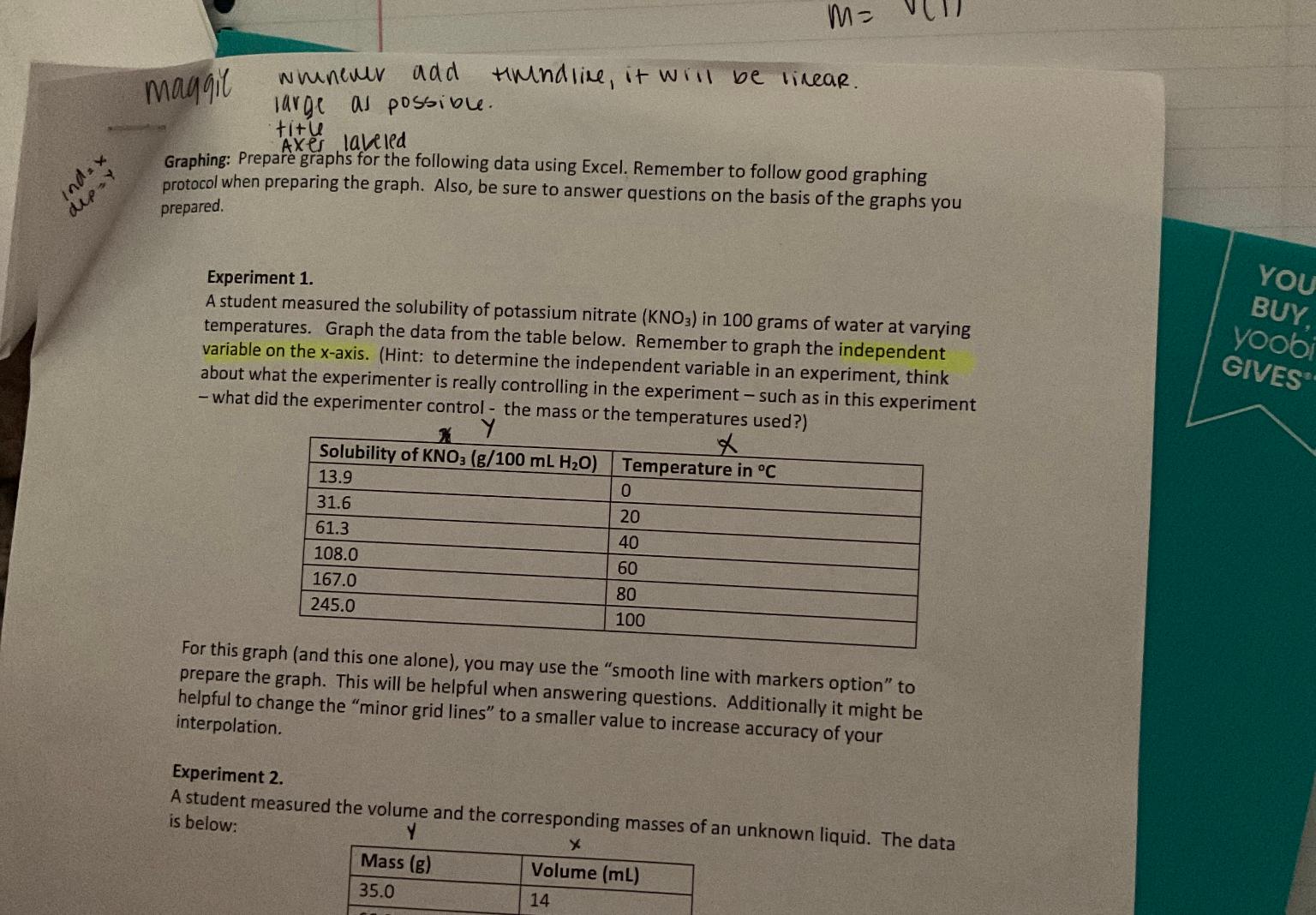
Graphing: Prepare graphs for the following data using Excel. Remember to follow good graphing protocol when preparing the graph. Also, be sure to answer questions on the basis of the graphs you prepared.
Experiment
A student measured the solubility of potassium nitrate in grams of water at varying temperatures. Graph the data from the table below. Remember to graph the independent variable on the axis. Hint: to determine the independent variable in an experiment, think about what the experimenter is really controlling in the experiment such as in this experiment what did the experimenter control the mass or the temperatures used?
table
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


