Question
The first step in the Ostwald process for the commercial production of nitric acid is carried out at 825 C. The reaction is 4NH3(g)
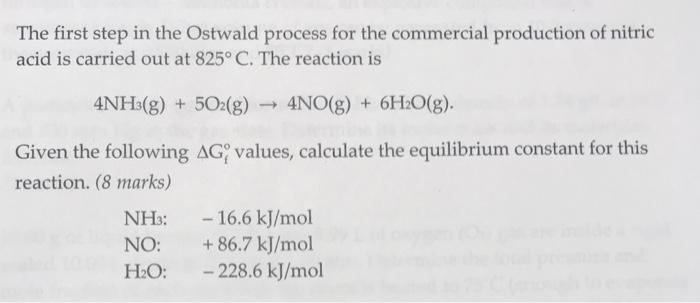
The first step in the Ostwald process for the commercial production of nitric acid is carried out at 825 C. The reaction is 4NH3(g) + 5O2(g) 4NO(g) + 6HO(g). Given the following AG values, calculate the equilibrium constant for this reaction. (8 marks) NH3: NO: HO: - 16.6 kJ/mol + 86.7 kJ/mol - 228.6 kJ/mol
Step by Step Solution
3.40 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
4NH3 9 Alloxn So 50 9 AGrx AG Product AGreoctont AAG...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Principles of Auditing and Other Assurance Services
Authors: Ray Whittington, Kurt Pany
19th edition
978-0077804770, 78025613, 77804775, 978-0078025617
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



