Question
AGF,298 values for BaSO4(s), Ba2+(aq), and SO42-(aq) are -1362.31, -560.74, and -744.63 kJ/mol, respectively. a) Find Ksp for BaSO4 in water at 25 C.
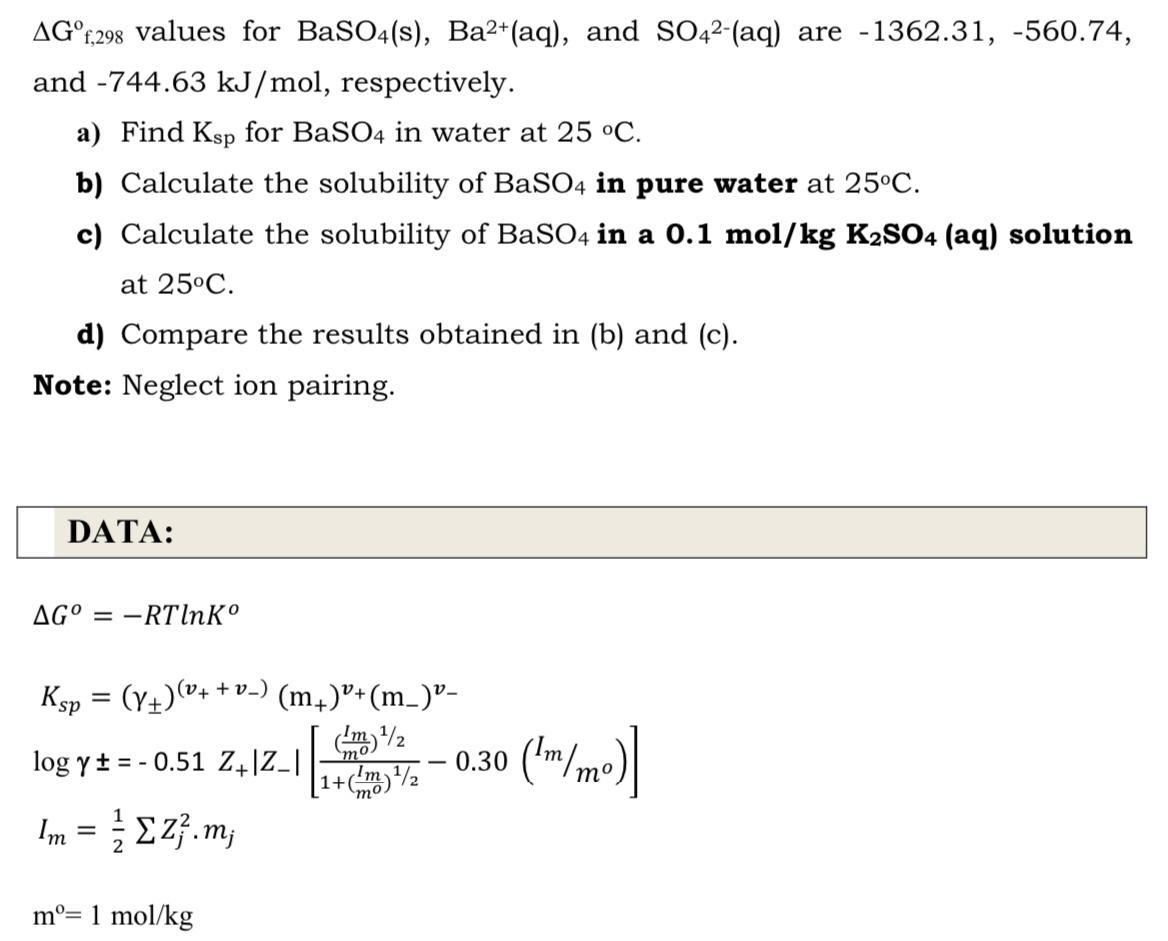
AGF,298 values for BaSO4(s), Ba2+(aq), and SO42-(aq) are -1362.31, -560.74, and -744.63 kJ/mol, respectively. a) Find Ksp for BaSO4 in water at 25 C. b) Calculate the solubility of BaSO4 in pure water at 25C. c) Calculate the solubility of BaSO4 in a 0.1 mol/kg K2SO4 (aq) solution at 25C. d) Compare the results obtained in (b) and (c). Note: Neglect ion pairing. DATA: AG = -RTlnK (v4 + v_) Ksp = (Y+)(+ (m,)"+(m_)"- log yt = - 0.51 Z4]Z_| 0.30 ('m/mo)| mo Im = E z}.m; %3D m= 1 mol/kg
Step by Step Solution
3.37 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



