Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A)How much MP and BP steam are consumed in total at the mill? Give the answer in MWs. Remember to compensate the heat losses in
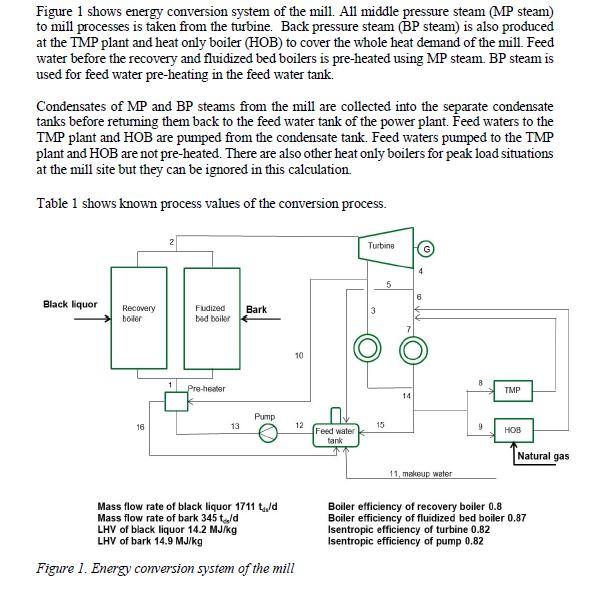
A)How much MP and BP steam are consumed in total at the mill? Give the answer in MWs. Remember to compensate the heat losses in this calculation by the make-up water. B) How much electricity does the turbine produce? Mechanical efficiency of the turbine is 0.98 and the generator efficiency is 0.97 and Calculate how much heat the CHP plant produces in MWs
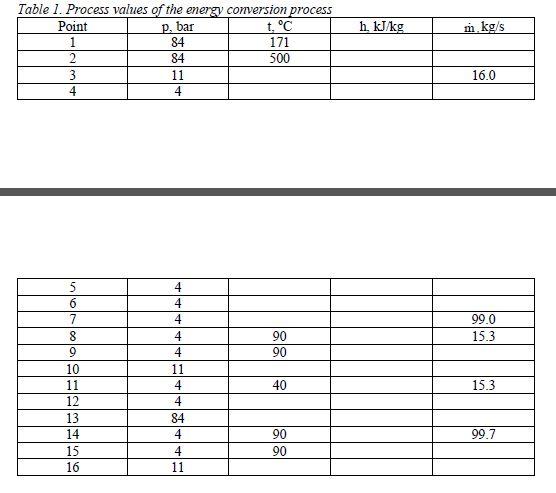
Figure 1 shows energy conversion system of the mill. All middle pressure steam (MP steam) to mill processes is taken from the turbine. Back pressure steam (BP steam) is also produced at the TMP plant and heat only boiler (HOB) to cover the whole heat demand of the mill. Feed water before the recovery and fluidized bed boilers is pre-heated using MP steam. BP steam is used for feed water pre-heating in the feed water tank. Condensates of MP and BP steams from the mill are collected into the separate condensate tanks before returning them back to the feed water tank of the power plant. Feed waters to the TMP plant and HOB are pumped from the condensate tank. Feed waters pumped to the TMP plant and HOB are not pre-heated. There are also other heat only boilers for peak load situations at the mill site but they can be ignored in this calculation. Table 1 shows known process values of the conversion process. Mass flow rate of black liquor 1711Lds/d Boiler efficiency of recovery boiler 0.8 Mass flow rate of bark 345tss/d Boiler efficiency of fluidized bed boiler 0.87 LHV of black liquor 14.2MJ/kg Isentropic efficiency of turbine 0.82 LHV of bark 14.9MJ/kg Isentropic efficiency of pump 0.82 Figure 1. Energy conversion system of the mill Table 1. Process values of the energy conversion process \begin{tabular}{|c|c|c|c|c|} \hline Point & p, bar & t,C & h,kJ/kg & m,kg/s \\ \hline 1 & 84 & 171 & & \\ \hline 2 & 84 & 500 & & \\ \hline 3 & 11 & & & 16.0 \\ \hline 4 & 4 & & & \\ \hline \end{tabular} \begin{tabular}{|c|c|c|c|c|} \hline 5 & 4 & & & \\ \hline 6 & 4 & & & \\ \hline 7 & 4 & & & 99.0 \\ \hline 8 & 4 & 90 & & 15.3 \\ \hline 9 & 4 & 90 & & \\ \hline 10 & 11 & & & \\ \hline 11 & 4 & 40 & & \\ \hline 12 & 4 & & & \\ \hline 13 & 84 & & & \\ \hline 14 & 4 & 90 & & \\ \hline 15 & 4 & 90 & & \\ \hline 16 & 11 & & & \\ \hline \end{tabular}Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


