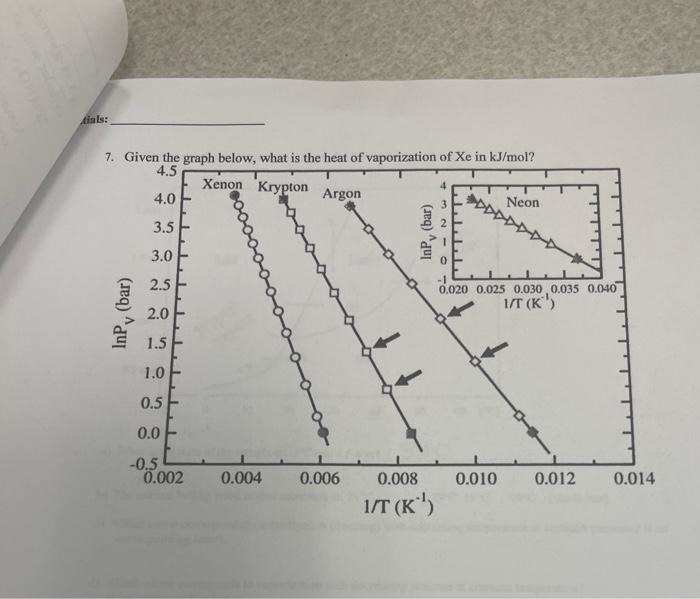
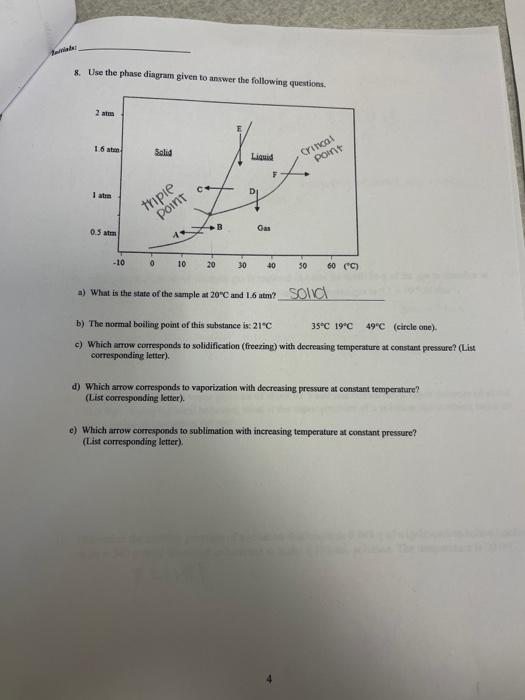
Ainals: 7. Given the graph below, what is the heat of vaporization of Xe in kJ/mol ? 9. A solution contsining 306.5g of Mgy(NO3)per liter has a density of 1.114gimL. a) The molarity of the solution is: 5.3050 molarity =11tticisorsolumionmolesorsolure=2.072 99446 349 /) 4 the molality of the solution is: mulaily =4gofsoutionmolesoksolute mutiply vowme by mass 10. What partial pressure of nitrogen gas is required in order for 0.00134 g of the gas to disolve in 13.1 mL. of pure water? The Henry's law constant for nitrogen gas is 6.1104Matm1. 29=Lmoles. 11. At 40C, heptane has a vapor pressure of about 91.5 totr and octane has a vapor pressure of about 31.2 torr. Assuming ideal behavior, what is the vapor pressure of a solution that contains twice as many moles of heptane as octane? 12. Determine the osmotic pressure of a solution that contains 0.048g of a hydrocarbon solute (molar mass =340g/mol ) dissolved in benzene to make a 350mL solution. The temperature is 20.0C. =IMRT 9. A solution contsining 306.5g of Mgy(NO3)per liter has a density of 1.114gimL. a) The molarity of the solution is: 5.3050 molarity =11tticisorsolumionmolesorsolure=2.072 99446 349 /) 4 the molality of the solution is: mulaily =4gofsoutionmolesoksolute mutiply vowme by mass 10. What partial pressure of nitrogen gas is required in order for 0.00134 g of the gas to disolve in 13.1 mL. of pure water? The Henry's law constant for nitrogen gas is 6.1104Matm1. 29=Lmoles. 11. At 40C, heptane has a vapor pressure of about 91.5 totr and octane has a vapor pressure of about 31.2 torr. Assuming ideal behavior, what is the vapor pressure of a solution that contains twice as many moles of heptane as octane? 12. Determine the osmotic pressure of a solution that contains 0.048g of a hydrocarbon solute (molar mass =340g/mol ) dissolved in benzene to make a 350mL solution. The temperature is 20.0C. =IMRT 4. N 3.31-g ample of load nitrate, Pb(NO3)2+ molar mass =331 gimot is heated in an evacuated cylinder with a volume of 2.37L. The salt decomipones when heated, according to the equation: Assuming complete decomposition, what is the pressure in the cylinder afler decomposition and 5. A gaseous mixture containing. 1.5mol Ar and 3.5molCO2 has a total pressure of 7.3atm. What is. the partial pressure of CO2 ? 1.5 mol Ar 3.5m ol :CO Given 6. How much enengy is needed to convert 54,1 grams of ice at 0.009C to water at 75.0C ? specifie beat (ice) 2.10JgCg=m) Cs (TrT 8. Use the phase diagram given to anwwer the following questions. a) What is the state of the smple at 20C and 1.6atm ? b) The nommal boiliag point of this substance is: 21C35C19C49C (circle one). c) Which arross corresponds to solidification (freezing) with decreasing termperature at constant pressure? (List corresponding Jetter). d) Which arrow corresponds to vaporiation with decreasing pressure at constant temperature? (List cocrespoeding letter). e) Which arrow comesponds to sublimation with increasing temperature at constant pressure? (List correspanding letter)