Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Air at atmospheric pressure is blown over a Cu-rich coppergold liquid solution at 1200 oC. If only the copper is oxidized (to form pure solid
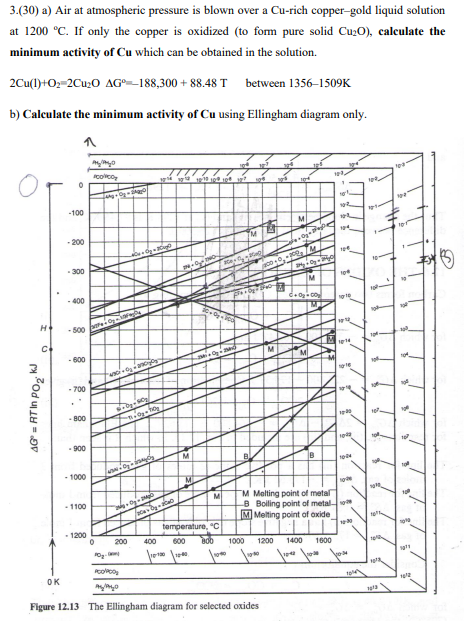
Air at atmospheric pressure is blown over a Cu-rich coppergold liquid solution at 1200 oC. If only the copper is oxidized (to form pure solid Cu2O), calculate the minimum activity of Cu which can be obtained in the solution.
3.(30) a) Air at atmospheric pressure is blown over a Cu-rich copper-gold liquid solution at 1200C. If only the copper is oxidized (to form pure solid Cu2O ), calculate the minimum activity of Cu which can be obtained in the solution. 2Cu(1)+O2=2Cu2OG=188,300+88.48T between 13561509K b) Calculate the minimum activity of Cu using Ellingham diagram only. 1. rqure lals 1 ne Eingnam aragrarn aor sevccted uxucsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


