Question
Air confined to one side of a rigid container divided by apartition, as shown in Fig.2. The outer side is initiallyevacuated. The air is initially
Air confined to one side of a rigid container divided by apartition, as shown in Fig.2. The outer side is initiallyevacuated. The air is initially at p1=5 bar,T1=500 K, and V1= 0.2m3. When the partition is removed, the air expands to fill the entirechamber. Measurements show that V2=2V1and p2=p1/4. Assuming the air behavesas an ideal gas, determine (a) the final temperature, in K, and (b)the heat transfer, kJ.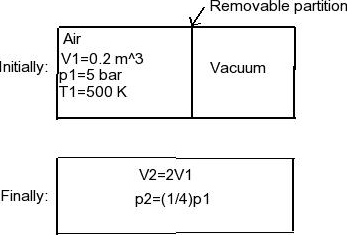
Air Removable partition V1=0.2 m^3 Initially: p1=5 bar T1=500 K Vacuum Finally: V2=2V1 p2=(1/4)p1
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Interactive Approach
Authors: Subrata Bhattacharjee
1st edition
130351172, 978-0130351173
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



