Question
Air enters a compressor at 100 kPa and 25 C. The air is compressed to 1MPa where it exits the compressor at 540 K.
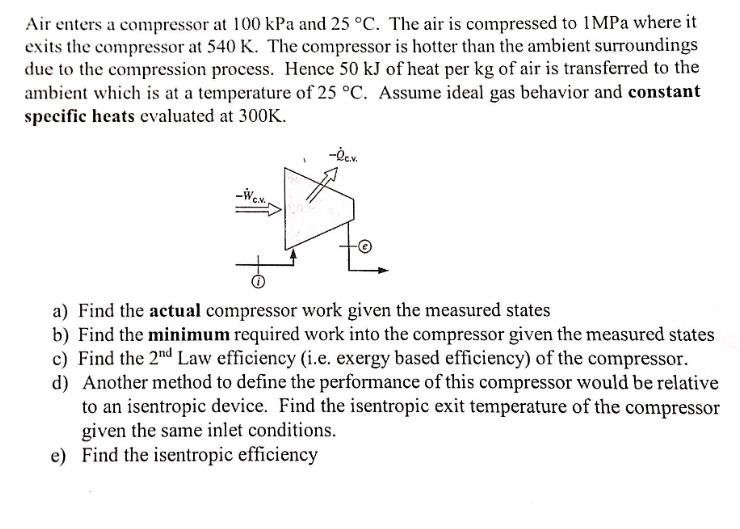
Air enters a compressor at 100 kPa and 25 C. The air is compressed to 1MPa where it exits the compressor at 540 K. The compressor is hotter than the ambient surroundings due to the compression process. Hence 50 kJ of heat per kg of air is transferred to the ambient which is at a temperature of 25 C. Assume ideal gas behavior and constant specific heats evaluated at 300K. -ec.V. * a) Find the actual compressor work given the measured states b) Find the minimum required work into the compressor given the measured states c) Find the 2nd Law efficiency (i.e. exergy based efficiency) of the compressor. d) Another method to define the performance of this compressor would be relative to an isentropic device. Find the isentropic exit temperature of the compressor given the same inlet conditions. e) Find the isentropic efficiency
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Engineering Approach
Authors: Yunus A. Cengel, Michael A. Boles
8th edition
73398179, 978-0073398174
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



