Answered step by step
Verified Expert Solution
Question
1 Approved Answer
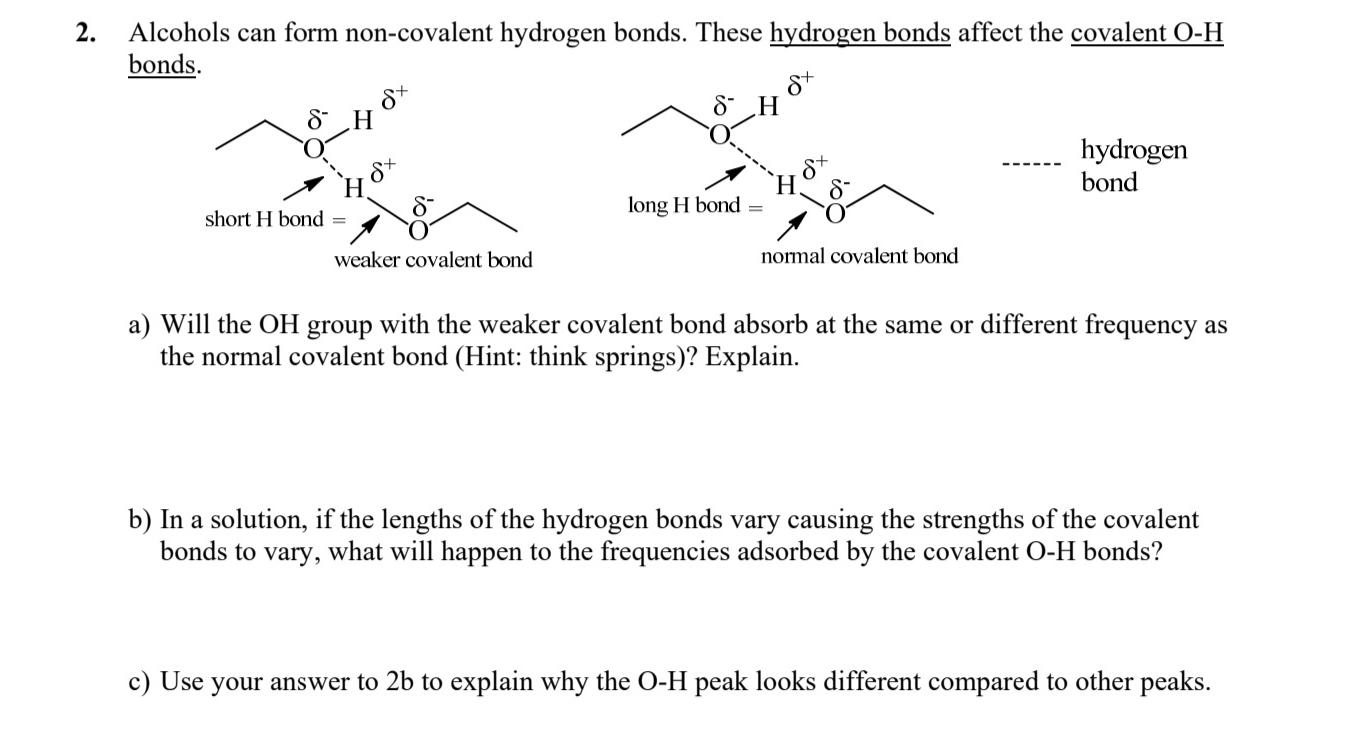
Alcohols can form non - covalent hydrogen bonds. These hydrogen bonds affect the covalent O - H bonds. hydrogen bond a ) Will the O
Alcohols can form noncovalent hydrogen bonds. These hydrogen bonds affect the covalent bonds.
hydrogen bond
a Will the group with the weaker covalent bond absorb at the same or different frequency as the normal covalent bond Hint: think springs Explain.
b In a solution, if the lengths of the hydrogen bonds vary causing the strengths of the covalent bonds to vary, what will happen to the frequencies adsorbed by the covalent bonds?
c Use your answer to to explain why the peak looks different compared to other peaks.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


