Question
Alkenes can be converted to alcohols by reaction with mercuric acetate to form a ?-hydroxyalkylmercury(II) acetate compound, a reaction called oxymercuration. Subsequent reduction with NaBH4
Alkenes can be converted to alcohols by reaction with mercuric acetate to form a ?-hydroxyalkylmercury(II) acetate compound, a reaction called oxymercuration. Subsequent reduction with NaBH4 reduces the C-Hg bond to a C-H bond, forming the alkyl alcohol, a reaction called demercuration. Draw the structures of the Hg-containing compound(s) and the final alcohol product(s) formed in the following reaction sequence, omitting byproducts. If applicable, draw hydrogen at a chirality center and indicate stereochemistry via wedge-and-dash bonds.
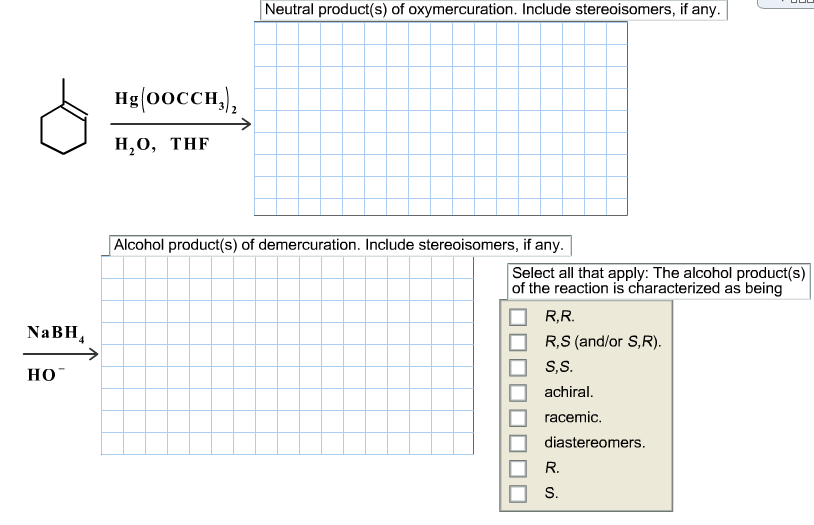
NaBH HO Hg(OOCCH) HO, THF Neutral product(s) of oxymercuration. Include stereoisomers, if any. Alcohol product(s) of demercuration. Include stereoisomers, if any. Select all that apply: The alcohol product(s) of the reaction is characterized as being R,R. R,S (and/or S,R). S,S. achiral. racemic. diastereomers. R. c S.
Step by Step Solution
3.52 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
Question 8 of 10 Incorrect Incorrect Solution omitting byproducts ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


