Alkenes can be converted into alcohols by acid-catalyzed addition of water. Assuming that Markovnikov?s rule is valid,
Question:
Alkenes can be converted into alcohols by acid-catalyzed addition of water. Assuming that Markovnikov?s rule is valid, predict the major alcohol product from each of the following alkenes.
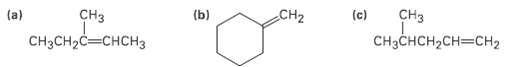
Transcribed Image Text:
сHз CH3CH-C—снсHз (b) CH2 CHз (a) (c) Cнаснсн2сH—сH2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (18 reviews)
a b c CH3 CH3CHCCHCH3 HO CH ...View the full answer

Answered By

John Aketch
I have a 10 years tutoring experience and I have helped thousands of students to accomplish their educational endeavors globally. What interests me most is when I see my students being succeeding in their classwork. I am confident that I will bring a great change to thins organization if granted the opportunity. Thanks
5.00+
8+ Reviews
18+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Predict the major organic product from each of the following reactions. (a) (b) (c) (d) (1) CH MgBr (2) HO (1) MgBr (2) NH,CI, H20 MgBr (1 equiv.) O (1) o (2) H,O (1) CH,CHLi (excess) (2) NH,CI, H2O O
-
Predict the major organic product from each of the following reaction sequences. (a) (b) (c) (d) (e) (f) (1) MeMgBr (excess) (2) NH,CI, H20 (1) Mg (2) H H (3) HyO (1) PBr3 (2) Mg (3) H,o OH OH (1)...
-
Predict the organic product from each of the following oxidation reactions. (a) (b) (c) (d) (e) (1) KMnO4, HO. OH (2) H,O OH PCC CH2Cl2 OH (1) DMSO, (COCI)2 (2) EtgN OH H2CrO H2CrO
-
Figure shows a cycle consisting of five paths: AB is isothermal at 300 K, BC is adiabatic with work = 5.0 J, CD is at a constant pressure of 5 atm, D E is isothermal, and EA is adiabatic with a...
-
What would a boss of yours have to do to demonstrate that he or she is an effective leader and an effective manager?
-
A sinusoidal message signal has a frequency of 250 Hz. This signal is the input to an FM modulator with an index of 8. Determine the bandwidth of the modulator output if a power ratio, P r , of 0.8...
-
47. LaMont works for a company in downtown Chicago. The company encourages employees to use public transportation (to save the environment) by providing them with transit passes at a cost of $270 per...
-
How much time does Janet have after filing the bankruptcy petition to submit the required schedules? What happens if Janet does not meet the deadline? Three months ago, Janet Harts husband of twenty...
-
Which of the following are NOT tax deductible expenses? a. expenses of a domestic nature b. expenses of a capital nature c. expenses incurred in earning exempt income d. all of the above
-
Determine which matrices in Exercise 2 are tri diagonal and positive definite. Repeat Exercise 2 for these matrices using the optimal choice of . In Exercise 2 a. 4x1 + x2 x3 = 5, x1 + 3x2 + x3 = 4,...
-
Aromatic compounds such as benzene react with alkyl chlorides in the presence of A1C1 3 catalyst to yield alkylbenzenes. The reaction occurs through a carbocation intermediate, formed by reaction of...
-
Reaction of 2, 3-dimcthyl-1-butenc with HBr leads to an alkyl bromide, C6H13Br. On treatment of this alkyl bromide with KOH in methanol, elimination of HBr to give an alkene occurs and a hydrocarbon...
-
A pressure vessel contains liquid water and water vapor in equilibrium at 350(oF). The total mass of liquid and vapor is 3(Ibm). If the volume of vapor is 50 times the volume of liquid, what is the...
-
Suppose a company bases its hourly rates on the number of customers per hour. The hourly rate the company charges is given by two functions where = g(2) 4, g(3) = 2, 9(4) = 3 and f(2) = 6, f(3) = 3,...
-
Which statements about insurance are true? 1- Insurance protects against the the worst-case scenario. All rational people want to buy insurance. 2- Insurance costs money, and therefore always...
-
need step by step instruction about creating this: in NX12 PART NAME: BRACKET ALL FILLETS R .313 ALL ROUNDS R .625 2X .500 1/500 2.875 9.500 4750 2875 $500 3.000 750 GENTERED IN OBJECT 2.375
-
8. Convert the angle - 7t from radian measure into degree measure. Show some work. 4
-
4. Variance Analysis. (CPA, adapted) The H. G. Company uses a standard cost system in accounting for the cost of one of its products. < The Budget is based on normal capacity of monthly production of...
-
The measures of cost performance are homogeneous; that is, all are expressed in terms of dollars rather than partly in dollars and partly in physical terms. This facilitates the summation of total...
-
suppose a nickel-contaminated soil 15 cm deep contained 800 mg/kg Ni, Vegetation was planted to remove the nickel by phytoremediation. The above-ground plant parts average 1% Ni on a dry-weight bas...
-
Explain the process of dynamic equilibrium. How is dynamic equilibrium related to vapor pressure?
-
Tell whether the following compound is chiral. trans- I,2-dimethylcyclopropane
-
Compare the difficulty of making models of trans-bicyclo[3.1.0]hexane and transbicyclo[ 5.3.0]decane. Which is easier to make? Explain.
-
Use models if necessary to help you decide which compound within each pair should have the greater heat of formation. Explain.
-
TB SA Qu. 13-74 (Static) What must Abdu invest today to... What must Abdu invest today to receive an annuity of $9,000 for four years semiannually at an 8% annual rate? All withdrawals will be made...
-
The tolal landed coet with the order gaantly sire of 6,000 unts is 4 (Enter your response roundod to the nearest dolar)
-
Boyne Inc. had beginning inventory of $12,000 at cost and $20,000 at retail. Net purchases were $120,000 at cost and $170,000 at retail. Net markups were $10,000, net markdowns were $7,000, and sales...

Study smarter with the SolutionInn App


