Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Alkyl chloride chemistry is extremely useful in the production of many products for a wide variety of applications, from pharmaceuticals to plastics. Of particular
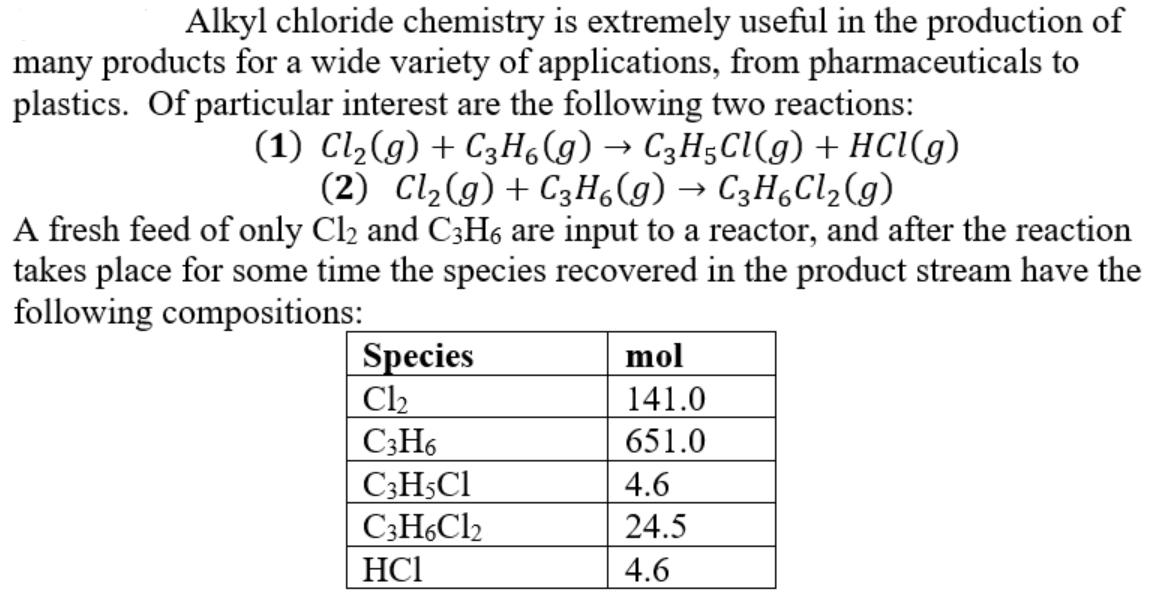
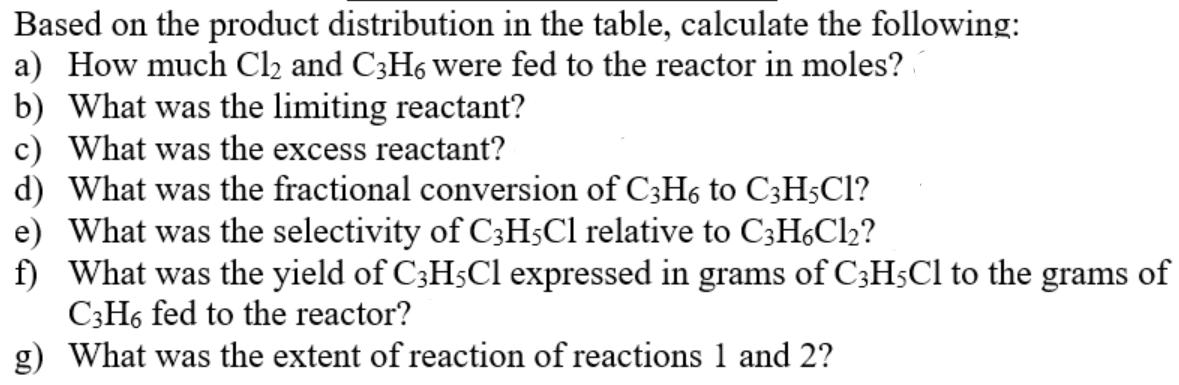
Alkyl chloride chemistry is extremely useful in the production of many products for a wide variety of applications, from pharmaceuticals to plastics. Of particular interest are the following two reactions: (1) Cl(g) + C3H6(g) C3H5Cl(g) + HCl(g) (2) Cl(g) + C3H6(g) C3H6Cl(g) A fresh feed of only Cl2 and C3H6 are input to a reactor, and after the reaction takes place for some time the species recovered in the product stream have the following compositions: Species Cl C3H6 C3H5C1 C3H6C12 HC1 mol 141.0 651.0 4.6 24.5 4.6 Based on the product distribution in the table, calculate the following: a) How much Cl2 and C3H6 were fed to the reactor in moles? b) What was the limiting reactant? c) What was the excess reactant? d) What was the fractional conversion of C3H6 to C3H5C1? e) What was the selectivity of C3H5Cl relative to C3H6Cl? f) What was the yield of C3H5C1 expressed in grams of C3H5Cl to the grams of C3H6 fed to the reactor? g) What was the extent of reaction of reactions 1 and 2?
Step by Step Solution
★★★★★
3.51 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
a The moles of Cl 2 and C 3 H 6 fed to the reactor can be calculated by considering the species compositions in the product stream Moles of Cl 2 fed 1...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


